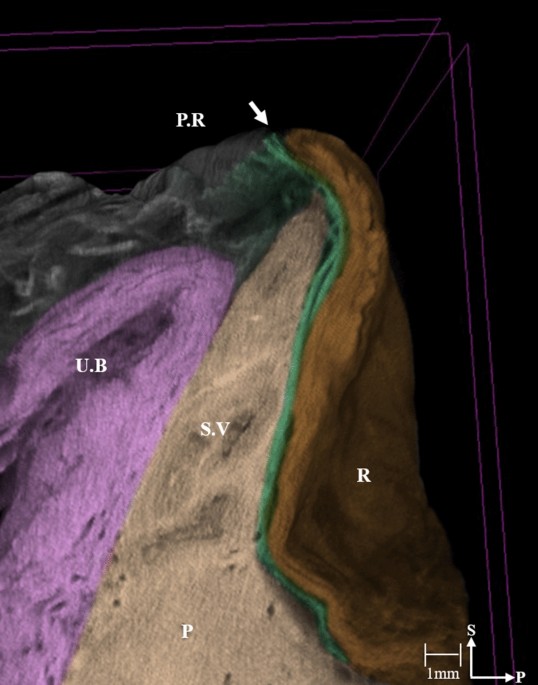
In the present study, images obtained using micro-CT and Masson’s trichrome staining showed that the DVF consists of a multilayered structure containing a mixture of collagenous, connective, and elastic tissues with smooth muscle fibers separating the rectum from the seminal vesicles and the prostate. Moreover, the urogenital NVB at the posterolateral corner of the prostate was separated from the mesorectum.
The DVF is an essential surgical landmark because, regardless of the direction of the dissection, it is associated with urogenital dysfunction as well as oncologic outcomes during TME, a standard surgery for patients with rectal cancer. It is crucial to understand the anatomy of this complex and thin structure accurately because it is challenging to identify the DVF in clinical practice, even if surgery is performed laparoscopically or robotically, which commonly provides a better surgical view.
TME respects the embryonic plane and involves an en-bloc resection of the tumor and its surrounding mesorectum via a sharp dissection of the visceral plane from the parietal fascia to secure oncologic safety16,17. However, in contrast to improvements in oncologic outcomes, the incidence of urogenital dysfunction remains high due to pelvic autonomic nerve (PAN) injury during surgery17,18,19. Thus, the relationship between the DVF, the MRF, and the NVBs has been studied, particularly in male patients with rectal cancer. Much controversy in the literature concerns the origin and development of the fascia, which has been described as either fused embryonic layers or compressed multiple layers of mesenchymal tissue to form a multilayered structure10. Recent studies have described the DVF as a multilayered, membranous, fascial structure instead of a single, membranous layer located behind the prostate and between the seminal vesicles and the rectum that developed from the fusion of the embryonic peritoneum of the rectovesical cul-de-sac, the original description of Charles-Pierre Denonvilliers1,2,5,7,12.
In the present study, the micro-CT images reveal the DVF as a continuous thin plate with multiple layers of condensed medial and pan-shaped dispersed lateral portions. The DVF can be distinguished from the MRF in its lateral portion, but multiple layers appear compressed with the MRF, with the two fasciae indistinguishable in the medial region. These features are commonly observed in the peritoneal reflection, at the level of the seminal vesicles, including the fusion of the DVF with the prostatic fascia. These findings support those of previous studies describing the DVF as a multilayered, membrane-like structure with the DVF and the MRF as distinctive layers, indicative of an obvious surgical plane between them1,2,5,7,12. Furthermore, these findings provide evidence that the posterior DVF should be an adequate dissection plane during the performance of an anterior TME without violating the key principle that the MRF should be preserved intact unless the tumor is locally advanced or anteriorly located.
Originally, Heald et al. reported that the correct TME plane should be anterior to the DVF to secure a safe, circumferential resection margin, as the DVF is part of the mesorectum’s anterior surface; these authors stressed that the surgeon should identify and preserve both edges of the DVF with a U-shaped incision to avoid postoperative urogenital dysfunction8,9. With Masson’s trichrome stain, we confirmed that the PAN was located between the DVF and the seminal vesicles and prostate even though the distribution of the PAN decreased in the medial region of the DVF. It is well known that the PAN extends immediately in front of the DVF to communicate with the bilateral pelvic plexuses; thus, using a surgical plane anterior to the DVF may cause erectile dysfunction in males20. Our micro-CT images and histologic findings are consistent with the results of previous studies in that at the prostate level, the lateral continuation of the DVF separates the urogenital NVB from the MRF1,11. Furthermore, we observed that the multiple layers of the DVF divide the posterior sheet covering the rectum, while the anterior sheet forms a tight fibrous plate that is firmly joined to the prostatic fascia. These findings suggest that to preserve the NVBs, the dissection plane should be posterior to the DVF, as the DVF is fused with the prostatic fascia; otherwise, the use of a dissection plane anterior to the DVF may damage the NVB (Fig. 5).
Figure 5 Schematic representation of the relationships of the correct surgical plane during an anterior total mesorectal excision to the Denonvilliers’s fascia in the male. The authors would like to thank Dong-Su Jang, MID (Medical Illustration & Design), a part of the Medical Research Support Services of sei University College of Medicine, for all artistic support related to this work. Full size image
A previous study evaluating the relationship between the DVF and the MRF suggested that the optimal TME plane is anterior to the DVF. These authors failed to distinguish the DVF from the MRF, even at high microscopic magnification11. We believe that this discrepancy is due to technical limitations from the inevitable alterations of the local tissue during the processing of the specimens. To avoid this, we used micro-CT imaging, which does not alter the morphological relationship between the DVF, the MRF, and the NVBs.
To the best of our knowledge, this is the first study to use a nondestructive morphological method combined with histological observations of fresh cadaver tissue to identify the anatomic relationship between the DVF, the MRF, and the NVBs. Among several agents that can be used to enhance the contrast of the soft tissue, we used PTA as it produces high-contrast X-ray images in a wide variety of soft tissues14. Using a nondestructive morphological method, PTA staining can avoid altering the original configuration of the soft tissues during manual dissection as the fasciae are close to each other21.
Our study has some limitations. First, a relatively small number of specimens were evaluated, although this issue was unavoidable in light of the few cadavers available. Second, even though fresh cadavers were used, the specimens were collected from elderly cadavers. Fibrous tissues may have been altered due to degeneration of the prostate and seminal vesicles with age. Finally, data comparing the urogenital function in patients with rectal cancer in whom the dissection was performed anterior or posterior to the DVF are not provided. Therefore, a well-designed randomized controlled trial evaluating the optimal dissection plane for TME is needed.
Based on our micro-CT images and Masson’s trichrome staining results, the DVF appears as a multilayered structure that separates the rectum from the seminal vesicles, as well as the urogenital NVBs from the mesorectum at the posterior corner of the prostate. To preserve the PAN for urogenital function and optimal oncologic outcomes, the optimal dissection plane for TME in patients with rectal cancer should be located posterior to the DVF.
http://feeds.nature.com/~r/srep/rss/current/~3/SEyzKyMMttc/s41598-021-01106-8