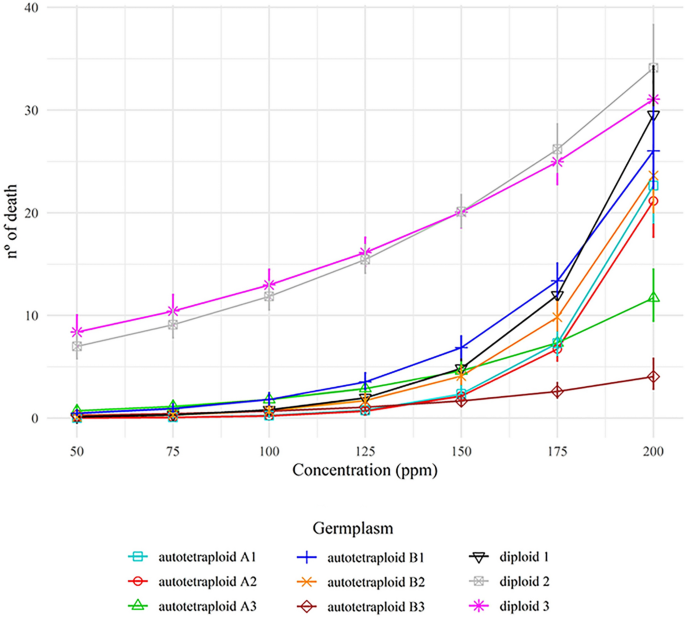
Short-term changes related to autotetraploidy in essential oil composition of Eucalyptus benthamii Maiden & Cambage and its applications in different bioassays Some forest trees have been polyploidized to improve their traits and to supply new germplasms for breeding programs. As trees have a long juvenile stage, the early characterization of the chromosome set doubling effects is crucial for previous selection. Thus, we aimed to characterize the chemical variability of essential oils from diploid and autotetraploid germplasms (autotetraploid A and B) of Eucalyptus benthamii, as well as to evaluate their larvicidal and allelopathic effects. Autotetraploid A showed a higher essential oil yield than diploid and autotetraploid B, which did not differ quantitatively. Aromadendrene, viridiflorol and α-pinene were the major compounds in the diploid essential oil. In contrast, compounds were present in autotetraploids, such as 1,8-cineole, limonene, α-terpineol, and α-terpinyl-acetate. Essential oils from the diploid at 50–200 ppm were twice as larvicidal than those from autotetraploids against Aedes aegypti larvae. Considering the phytotoxicity bioassays using Lactuca sativa, essential oils from both ploidy levels affected root growth. Moreover, the essential oils inhibited shoot growth at all concentrations tested (187.5; 375; 750; 1500; and 3000 ppm). Autotetraploid A and B had the same effect on shoot growth as glyphosate. The essential oils had no cytogenotoxic effect on root meristematic cells of L. sativa, whereas phytotoxic potential was identified mainly in shoot growth. This work demonstrated a dramatic change in secondary metabolism (terpene composition) related to an increase in the ploidy level in Eucalyptus germplasms. In addition, we report the novelty of the chemical composition of essential oils among germplasms and their potential use as larvicidal and post-emergence weed control agents. Eucalyptus is an important genus among the cultivated woody angiosperms, and it includes more than 700 species of trees and shrubs1. Eucalyptus belongs to the Myrtaceae Family, which is prolific in species that produce essential oils (EOs)2. From more than 300 different EOs of commercial value, at least 20 are found in the genera Eucalyptus and Corymbia, which possess species characterized by high EO production3.Exploration of Eucalyptus EOs dates from the nineteenth century in Australia4. Since then, advances in silviculture have been optimized to maximize EO production, such as selection and crossing involving superior genotypes5. Breeders were able to select important phenotypic features to improve EO yield and tree adaptation to different environments in some species, such as Corymbia citriodora (Hook.) K.D. Hill & L.A.S. Johnson, Eucalyptus camaldulensis Dehnh., Eucalyptus radiata D. C., Eucalyptus globulus Labill., and Eucalyptus staigeriana F. Muell6. Phytochemically, Eucalyptus EOs can have citronellal, citronellol, 1,8-cineole, α-pinene, α-terpineol, limonene, geranyl acetate and linalool, among other major compounds7. The chemical components, as well as the yield of EOs, may vary according to the environment. For example, E. camaldulensis from Argentina contains 1,8-cineole, p-cymene, β-phellandrene, and 0.38% EO yield, while in Brazil, the same species has 1,8-cineole, limonene, γ-terpinene, α-pinene, and 0.63% yield8,9. Despite the characterization of EO from E. benthamii leaves10, these trees are mostly planted for the production of cellulose, timber, or bioenergy. For example, leaves are left in the field as litter for biomass decomposition and have not yet been explored for EO extraction.Aiming for germplasm diversity, synthetic autotetraploid and allotetraploid Eucalyptus have been produced to be incorporated in breeding programs and in the field11,12. As the Eucalyptus nuclear genome potentially has 113 genes related to terpene synthesis13, chromosome set doubling (CSD) procedures increase the gene copy and, consequently, increase the phenotypic diversity of EO production and composition14,15. Therefore, the gene copy increases alleles per locus, generating more overdominance than in diploid counterparts16. The response for this effect covers the ‘omics’ (genomic, epigenomic, transcriptomic, and metabolomic), resulting in different yield and composition of secondary metabolites, such as EO17. In spite of CSD could enhance EO production, a careful screening and selection still needed among the autopolyploid or allopolyploid germplasms, or both, based on chemical profiling15,18,19.EO synthesis plays a central role in plants, providing defense against biotic and abiotic stresses20. Due to their chemical variability and toxicity, EOs are biomolecules extensively used as pesticides to control insect pests, plant diseases, and weeds7,8,10,21. Neotropical countries, such as Brazil, have suffered from dengue, zika, yellow fever, and chikungunya epidemics, transmitted by Aedes aegypti L.27. To control A. aegypti, health agencies frequently use pyrethroids as insecticides, which have larvicidal effect at concentrations as low as 1 ppm. However, this control method can promote insect resistance and affect non-target organisms23,24. Likewise, synthetic herbicides are applied indiscriminately and has caused problems to the environment and other organisms. Thereby, compounds of natural origin are considered promising in the obtention of novel pesticides that cause less impact25,26.In the present study, we described and compared the chemical composition of foliar EOs of one diploid and two synthetic autotetraploid E. benthamii germplasms. Additionally, we tested the larvicidal and allelopathic effects of these EOs. We used Aedes aegypti and Lactuca sativa L. in toxicity bioassays. Allelopathic effects of EOs were described by reducing seed germination, rate germination, as well as shoot and root growth of plantlets. The identification of aneugenic and clastogenic effects (such as chromatin condensation, micronuclei, and chromosome loss and alterations) was also used as a parameter to determine the toxicity of EOs. Moreover, flow cytometry analysis was used to verify the effect of EOs on cell proliferation.Seeds of E. benthamii from open-pollinated crosses were germinated, and a diploid germplasm (2n = 2 × = 22 chromosomes) was obtained and multiplied. The seed lot was licensed and provided by Klabin S.A. Solid autotetraploid plantlets (2C = 1.22 pg, 2n = 4 × = 44 chromosomes) were generated from two diploid plantlets submitted to the CSD procedure, using 1,5 mM of colchicine for 36 h of treatment exposure12. Considering that Eucalyptus is predominantly allogamous and the germplasms were generated from two distinct seeds of E. benthamii, the autotetraploids were denominated here as autotetraploid A and autotetraploid B. Diploid and autotetraploids were vegetative propagated and developed during 2016 and 2017 at the Laboratório de Citogenética e Citometria (Universidade Federal de Viçosa, MG, Brazil). The multiplication media used was 4.3 g L−1 MS basal salts (Sigma®) supplemented with 0.5 mg L−1 6-benzylaminopurine (Sigma®) and 0.05 mg L − 1 α-naphthaleneacetic (Sigma®). The ploidy level stability was checked by flow cytometry during in vitro propagation12.After three months of in vitro multiplication, the plantlets were acclimatized and transferred to the Klabin S.A. Experimental Research Station, Paraná, Brazil (24°13′31″S, 50°32′44″O). The trees were cultivated for 1.5 years and spaced 3 × 2 m, until they reached 5-m high and 12.5-m circumference at breast height. In the field, a new ploidy assessment was performed to check the DNA ploidy level stability. Leaves from nine trees (three from each germplasm) were collected during Autumn (April/May 2019), in three different portions along the longitudinal axis: basal, medium, and top of the crown. For harvesting, trees were cut and processed within 30 min each, 500 g of leaves were packed in kraft paper for each sample. After 24 h, the leaves were air-dried for seven days at 25 ± 1 °C, and weighed in an analytical balance to obtain the leaves’ dry mass. All samples were stored at − 20 °C until use27. The use of the plant material in this study is in accordance with Klabin S.A., national and international guidelines.EO extraction and yieldLeaves (50 g) from each sample were added to a round-bottom distillation flask (1 L) containing 600 mL of dH2O. The flasks were attached to a Clevenger apparatus and a condenser. Hydrodistillation was performed for 3 h, and the vapor of oils and water was condensed and collected into a 125 mL Erlenmeyer flask, as recommended by the Brazilian Phamacopoeia for volatile oils7,28. The hydrolate was subjected to liquid–liquid extraction using dichloromethane (3 × 10 mL). The organic phase was dried with anhydrous sodium sulfate, filtered, and concentrated under reduced pressure in a rotatory evaporator. The obtained EO was transferred to Eppendorff® microtubes and protected from the light with an aluminum foil, and stored at − 20 °C for 5 days. The EO yield was measured considering the dried mass of the leaves divided by the extracted EO mass in m m−17.Each sample from every germplasm was ranked according to EO yield (%) and denominated as 1 (lower yield), 2 (intermediate yield), and 3 (higher yield). The yield data were subjected to ANOVA and the Scott-Knott test at 5%, and a correlation analysis was performed between yield (%) and composition (%).Chromatographic profile of EOThe extracted EO of each sample was analyzed via gas chromatography with a flame ionization detector (GC-FID, Shimadzu GC-2010 Plus) and gas chromatography coupled to mass spectrometry (GC–MS, Shimadzu GCMS-QP2010 SE). EOs were removed from the vials in 1 μL of a solution consisting of 3% EOs dissolved in n-hexane. The analysis was conducted following the equipment conditions: Helium (He) as carrier gas in both detectors, with flow and linear speed of 2.80 mL min−1 and 50.8 cm s−1 (GC-FID), and 1.98 mL min−1 and 50.9 cm s−1 (GC–MS), respectively; 220 °C injector temperature in split ratio of 1:30; fused silica capillary column (30 m × 0.25 mm); stationary phase Rtx®-5MS (0.25 µm film thickness); oven program with initial temperature of 40 °C for 3 min followed by graduated increments of 3 °C min−1 up to 180 °C, which was maintained for 10 min for a total analysis time of 59.67 min; and 240 °C FID and 200 °C MS detector temperatures27,29. GC–MS analysis was performed in electronic impact equipment with 70 eV impact energy, 1000 scan speed, 0.50 fragment s−1 scanning interval, and fragment detection from 29 to 400 (m/z). GC-FID analysis was performed using an H2 flame and atmospheric air at 300 °C. Flow speeds used for H2 and air were 40 mL min−1 and 400 mL min−1, respectively.The identification of the EO components was performed by comparing the obtained mass spectra to those available in the spectral library database (Wiley 7, NIST 05, and NIST 05s) and the retention index (RI). To calculate the RI, a sample of saturated alkanes C7–C40 (Supelco, USA) and the adjusted retention time of each compound were employed as obtained by GC-FID. Subsequently, the values calculated for each compound were compared to those reported in the literature30. The percentage of each compound in the EO was calculated as the ratio between the integral area of the peaks and the total area of all sample constituents, as obtained from the GC-FID analysis. Compounds with a relative area > 1% were identified, and those > 5% were considered to be predominant.Larvicidal activityFor larvicidal activity, three EOs (1, 2, and 3) from diploids, autotetraploids A, and autotetraploid B were selected based on lower, intermediate, and higher yields (%). The phytotoxicity and cytotoxicity bioassays were performed with two EOs (lower and higher yields) of the diploid autotetraploids A and B.In order to verify the larvicidal activity of the EOs from diploids and autotetraploids E. benthamii, fourth-instar larvae of A. aegypti (PPCampos strain, Campos Goytacazes, RJ) were used. The larvae were obtained from a colony maintained at the Universidade Federal de Viçosa. The eggs were incubated in trays with dechlorinated (tap) water, kept under controlled conditions (26 ± 2 °C, 12 h L:D photoperiod), and fed with turtle food (Reptolife®, Alcon,Camboriú, SC, Brazil) until the L4 stage25. EO solutions were prepared with 1% dimethyl sulfoxide (DMSO, v v−1) at concentrations of 50, 75, 100, 125, 150, 175, and 200 ppm25. The larvae were collected with a Pasteur pipette and distributed into glass vials containing 30 mL of the EO solutions. Control solutions were prepared with 1% DMSO, as a negative control, and deltamethrin 0.07 ppm (Decis 25 EC, 25 g L−1, emulsifiable concentrate, Bayer S.A., Germany), as a positive control. The assay was performed with five repetitions for each treatment with 25 larvae each (125 larvae per treatment), without solution replacement; after 24 h, A. aegypti mortality was assessed. The larvae were considered dead when no response to stimuli was found (direct contact with a Pasteur pipette). Relationships between different EOs and their concentrations were evaluated with a regression analysis (9 oils × 7 concentrations). A generalized linear model was used, with a Poisson distribution of residual effect and logarithmic ligation function. The following factors EOs, concentration, and its interaction were evaluated with deviance analysis at 5% probability of type 1 error. Confidence intervals of 95% of probability were built for each concentration of EO with the Effects package31. All analyses were performed with R software32.Phytotoxicity bioassayA phytotoxicity bioassay was performed using EO concentrations tested at 187.5, 375, 750, 1500, and 3000 ppm33,34. The statistical design was completely randomized with 5 replications for each treatment. Each experimental unit consisted of a Petri dish (10 cm × 2 cm) containing 25 seeds of L. sativa ‘Itapuã Super’ (Isla S.A., Brazil), which were placed on a piece of filter paper. EOs were dissolved in dichloromethane and applied using a sterile Pasteur pipette. The evaluated variables after exposure to EOs were as follows: (a) germination rate (GR, %) and root growth (RG, mm) after 48 h of exposure; (b) germination speed index (GSI), calculated every 8 h during 48 h, using the formula (N1 . 1) + (N2 − N1) . 1/2 + (N3 − N2) . 1/3 + … (Ny − (Ny − 1)) . 1/y (where Ny: number of germinated seeds in a given period; Y: total number of evaluations); and (c) shoot growth (SG, mm), determined after 120 h of exposure. The phytotoxicity bioassay was analyzed through a multiple linear regression with dummy variables, comparing each variable with the following factors: negative controls (water and dichloromethane), po
https://www.nature.com/articles/s41598-021-03916-2
Short-term changes related to autotetraploidy in essential oil composition of Eucalyptus benthamii Maiden & Cambage and its applications in different bioassays