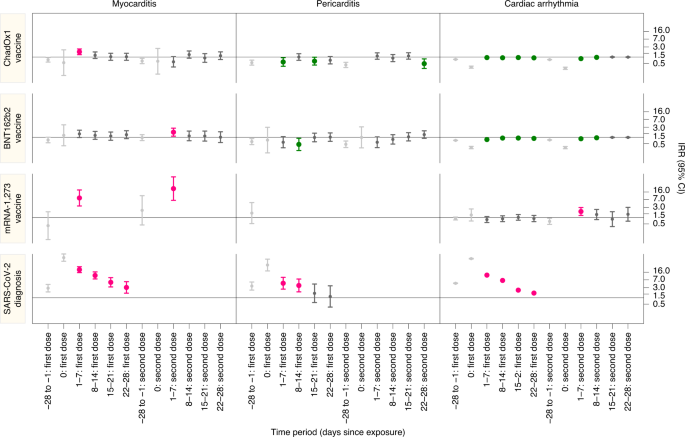
Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection Martina Patone1, Xue W. Mei orcid.org/0000-0002-6279-48841, Lahiru Handunnetthi orcid.org/0000-0002-8242-37222, Sharon Dixon1, Francesco Zaccardi3, Manu Shankar-Hari orcid.org/0000-0002-5338-25384,5,6, Peter Watkinson orcid.org/0000-0003-1023-39277,8, Kamlesh Khunti3, Anthony Harnden1, Carol A. C. Coupland orcid.org/0000-0002-2327-33061,9, Keith M. Channon10, Nicholas L. Mills orcid.org/0000-0003-0533-79914,11, Aziz Sheikh orcid.org/0000-0001-7022-30564 & Julia Hippisley-Cox orcid.org/0000-0002-2479-72831 Nature Medicine (2021)Cite this article Cardiovascular diseasesEpidemiology Although myocarditis and pericarditis were not observed as adverse events in coronavirus disease 2019 (COVID-19) vaccine trials, there have been numerous reports of suspected cases following vaccination in the general population. We undertook a self-controlled case series study of people aged 16 or older vaccinated for COVID-19 in England between 1 December 2020 and 24 August 2021 to investigate hospital admission or death from myocarditis, pericarditis and cardiac arrhythmias in the 1–28 days following adenovirus (ChAdOx1, n = 20,615,911) or messenger RNA-based (BNT162b2, n = 16,993,389; mRNA-1273, n = 1,006,191) vaccines or a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) positive test (n = 3,028,867). We found increased risks of myocarditis associated with the first dose of ChAdOx1 and BNT162b2 vaccines and the first and second doses of the mRNA-1273 vaccine over the 1–28 days postvaccination period, and after a SARS-CoV-2 positive test. We estimated an extra two (95% confidence interval (CI) 0, 3), one (95% CI 0, 2) and six (95% CI 2, 8) myocarditis events per 1 million people vaccinated with ChAdOx1, BNT162b2 and mRNA-1273, respectively, in the 28 days following a first dose and an extra ten (95% CI 7, 11) myocarditis events per 1 million vaccinated in the 28 days after a second dose of mRNA-1273. This compares with an extra 40 (95% CI 38, 41) myocarditis events per 1 million patients in the 28 days following a SARS-CoV-2 positive test. We also observed increased risks of pericarditis and cardiac arrhythmias following a positive SARS-CoV-2 test. Similar associations were not observed with any of the COVID-19 vaccines, apart from an increased risk of arrhythmia following a second dose of mRNA-1273. Subgroup analyses by age showed the increased risk of myocarditis associated with the two mRNA vaccines was present only in those younger than 40. By the end of September 2021, more than 6.3 billion doses of COVID-19 vaccination had been administered worldwide1. Clinical trials of COVID-19 vaccines were underpowered to detect the rare adverse events that are important for risk–benefit evaluations and to inform clinical practice postvaccination. Therefore, identifying such rare adverse events is now a global scientific priority.As of 4 November 2021, there have been 1,783 reports to the United States Vaccine Adverse Event Reporting System (VAERS) of cases of heart inflammation, namely myocarditis or pericarditis, among people aged 12–29 years who received COVID-19 vaccines, in particular following mRNA vaccination, that is, BNT162b2 and mRNA-1273 vaccines2. As of 9 July 2021, the European Medicines Agency (EMA) has reported 145 cases of myocarditis and 138 cases of pericarditis out of 177 million doses of the BNT162b2 vaccine, and 9 cases of myocarditis and 19 cases of pericarditis out of 20 million doses of the mRNA-1273 vaccine3. In Israel, 275 cases of myocarditis were reported between December 2020 and May 2021 among more than 5 million people vaccinated with the BNT162b2 vaccine4. No association between ChAdOx1 vaccine and myocarditis or pericarditis has been reported. The same reports showed that these events are more likely to occur in adolescent and young adults, mostly after the second dose. Evaluation of the risks of adverse events following vaccination or SARS-CoV-2 infection in different age groups provides crucial information to determine whether the risks from the vaccine outweighs the risks following a positive SARS-CoV-2 test.In England, the vaccination campaign began on 8 December 2020 with the BNT162b2 vaccine followed by the ChAdOx1 vaccine on 4 January 2021. In the first phase, priority was given to the most vulnerable, in a schedule based primarily on age. The mRNA-1273 vaccine became available in England on 13 April 2021. Since 7 April 2021, ChAdOx1 vaccine has not been recommended for individuals younger than 30 years of age, and since 7 May 2021 for individuals younger than 40 years of age.The English National Immunisation (NIMS) Database of COVID-19 vaccination includes data on vaccine type, date and doses for all people vaccinated in England. We linked NIMS, at individual patient level, to national data for mortality, hospital admissions and SARS-CoV-2 infection data to examine the associations between the first and second dose of ChAdOx1, BNT162b2 or mRNA-1273 vaccines and cardiac adverse events: myocarditis, pericarditis or cardiac arrhythmias. We used the same population to investigate the associations between a positive SARS-CoV-2 test (before or after vaccination) as a secondary exposure and the same cardiac adverse events. We also assessed risks for the same outcomes following vaccination or a SARS-CoV-2 positive test in younger persons (<40 years old). Incidence rate ratios, the rate of hospital admission or death from each outcome in risk periods after vaccination or a positive test relative to baseline periods, were estimated using self-controlled case series (SCCS) methodology5,6.A total of 38,615,491 adults had been vaccinated with at least one dose of ChAdOx1 (n = 20,615,911), BNT162b2 (n = 16,993,389) or mRNA-1273 (n = 1,006,191) in England between 1 December 2020 and 24 August 2021 (Table 1). Of these, 32,095,748 had received two doses of either ChAdOx1 (n = 19,754,224, 95.8%), BNT162b2 (n = 11,972,733, 70.5%) or mRNA-1273 (n = 368,791, 36.7%). Individuals receiving the ChAdOx1 and BNT162b2 vaccine were older, on average, than those receiving the mRNA-1273 vaccine, as expected given that the mRNA-1273 vaccine roll-out began in April 2021 in the United Kingdom, when higher priority risk groups (including older people) had already received their vaccine.Table 1 Baseline demographic characteristics of people receiving either ChAdOx1, BNT162b2 or mRNA-1273 vaccines or testing positive for SARS-CoV-2 virus (before or after vaccination), in England between 1 December 2020 and 24 August 2021. Data are presented as column % (counts)Amongst those with at least one dose, there were 3,028,867 (7.8%) individuals who had a SARS-CoV-2 positive test. Of these, 2,315,669 (6.0%) individuals tested positive before vaccination; while 713,198 (1.8%) and 298,315 (0.7%) tested positive after the first and second vaccine doses, respectively. Table 1 shows the characteristics of the study population, stratified by vaccine type and dose, and of those who tested positive for SARS-CoV-2.During the study period there were 1,615 and 1,574 admissions or deaths related to myocarditis and pericarditis, respectively (14 patients had both), and 385,508 related to cardiac arrhythmias. The characteristics of individuals with myocarditis, pericarditis and cardiac arrhythmias in the 1–28 days postvaccination differed by condition and according to the vaccine administered (Table 2). Supplementary Table 1 shows the characteristics of patients who died for the individual outcomes in the 1–28 days following a first or second dose of COVID-19 vaccine or SARS-CoV-2 infection. Table 3 and Fig. 1 show the number of patients with outcome events in each exposure time period and the incidence rate ratios (IRRs) and 95% CIs for outcomes in the exposure risk periods.Table 2 Demographic characteristics of patients who experienced the individual outcomes in the 1–28 days following a first or second dose of COVID-19 vaccine or SARS-CoV-2 infection amongst the vaccinated population in England from 1 December 2020 to 24 August 2021 (cells with an asterisk are suppressed)Table 3 IRR (95% CI) for individual outcomes in predefined risk periods immediately before and after exposure to vaccination and before and after a positive SARS-CoV-2 test result, adjusted for calendar time from 1 December 2020 to 24 August 2021 (cells with an asterisk are suppressed). n/a, not applicable; pyrs, person-yearsFig. 1: IRRs with 95% CIs for cardiac adverse events following each exposure.IRRs are presented for predefined risk periods (0, 1–7, 8–14, 15–21 and 22–28 days) after first or second dose of ChAdOx1, BNT162b2 and mRNA-1273 vaccines and a SARS-CoV-2 positive test for the prerisk period (28 days before exposure). Horizontal bold line in each panel indicates 1.MyocarditisOf the 38,615,491 vaccinated individuals included in our study, 1,615 (0.004%) were admitted to hospital with, or died from, myocarditis at any time in the study period (either before or after vaccination); 397 (0.001%) of these occurred in the 1-28 days post any dose of vaccine. Of the 1,615 who were admitted or died, 359 (22.2%) had a SARS-CoV-2 positive test, with 287 (17.8%) of these being before vaccination. There were 114 deaths with myocarditis recorded on the death certificate as a cause of death (23 had a SARS-CoV-2 positive test). Of those who have been admitted with, or died from, myocarditis in the 1-28 days postvaccination, 12.7% (18) and 10.7% (9) had a positive SARS-CoV-2 test before the first and second dose ChAdOx1 vaccine, respectively, and 7.4% (7) before the first dose of BNT162b2 vaccine (Table 2).There was an increased risk of myocarditis at 1–7 days following the first dose of ChAdOx1 (IRR 1.76; 95% CI 1.29, 2.42), BNT162b2 (IRR 1.45, 95% CI 0.97, 2.12) and mRNA-1273 (IRR 8.38, 95% CI 3.53, 19.91), and the second dose of BNT162b2 (IRR 1.75, 95% CI 1.13, 2.70) and mRNA-1273 (IRR 23.10, 95% CI 6.46, 82.56). There was an increased risk of myocarditis at 1–7 days (IRR 21.08, 95% CI 15.34, 28.96), 8–14 days (IRR 11.29, 95% CI 7.70, 16.57), 15–21 days (IRR 5.36, 95% CI 3.24, 8.89) and 21–28 days (IRR 3.08, 95%CI 1.65, 5.75) following a positive test.Over the 1–28 days postvaccination, we observed an association with the first dose of ChAdOx1 (IRR 1.29, 95% CI 1.05, 1.58), BNT162b2 (IRR 1.31, 95% CI 1.03, 1.66) and mRNA-1273 (IRR 2.97; 95% CI 1.34, 6.58). Following a second dose, the increased risk was much higher with mRNA-1273 (IRR 9.84, 95% CI 2.69, 36.03) compared with BNT162b2 (IRR 1.30, 95% CI 0.98, 1.72). The risk of myocarditis was increased in the 1–28 days following a SARS-CoV-2 positive test (IRR 9.76, 95% CI 7.51, 12.69).PericarditisOf the 38,615,491 vaccinated individuals included in our study, 1,574 (0.004%) were admitted to hospital with, or died from, pericarditis at any time in the study period (either before or after vaccination); 356 (0.001%) of these occurred in the 1-28 days after any dose of vaccine. Of the 1,574 who were admitted or died, 188 (11.9%) had a SARS-CoV-2 positive test, with 154 (9.8%) of these being before vaccination. There were 31 deaths with pericarditis recorded on the death certificate as cause of death (6 had a SARS-CoV-2 positive test). Table 2 shows the percentages of patients with pericarditis events in the risk period who had a positive SARS-CoV-2 test before vaccination by vaccine type and dose.There were reduced risks of pericarditis after a first dose of ChAdOx1 (IRR 0.59; 95% CI 0.37, 0.94 at 1–7 days, IRR 0.64; 95% CI 0.42, 0.99 at 15–21 days), of BNT162b2 (IRR 0.46; 95% CI 0.24, 0.90 at 8–14 days) and following a second dose of ChAdOx1 (IRR 0.49; 95% CI: 0.29, 0.82 at 22–28 days). There were insufficient numbers of events to evaluate associations with the mRNA-1273 vaccine by week. There was an increased risk of hospital admission or death for pericarditis at 1–7 days (IRR 4.85, 95% CI 2.56, 9.18) and 8–14 days (IRR 3.81, 95% CI 1.90, 7.63) following a SARS-CoV-2 positive test.Over the 1–28 days postvaccination, we observed a decreased risk of pericarditis following the first dose of ChAdOx1 (IRR 0.74, 95%CI 0.59, 0.92), in contrast with an increased risk in the 1–28 days following a SARS-CoV-2 positive test (IRR 2.79, 95% CI 1.80, 4.32). No association was observed with the BNT162b2 or mRNA-1273 vaccine.Cardiac arrhythmiaOf the 38,615,491 vaccinated individuals included in our study, 385,508 (1.0%) were admitted to hospital with or died from cardiac arrhythmia at any time in the study period (either before or after vaccination); 86,754 (0.2%) of these occurred in the 1-28 days after any dose of vaccine. Of those who were admitted or died 39,897 (10.3%) had a SARS-CoV-2 positive test, with 29,694 (7.7%) having a positive test before vaccination. There were 7,795 deaths with cardiac arrhythmia recorded as the cause of death (1,108 had a SARS-CoV-2 positive test). Table 2 shows the percentages of patients with cardiac arrhythmia events in the risk period who had a positive SARS-CoV-2 test before vaccination by vaccine type and dose.There were decreased risks of cardiac arrhythmia after the first dose of ChAdOx1 (IRR 0.95, 95% CI 0.92, 0.97 at 1–7 days and over subsequent periods) and BNT162b2 (IRR 0.79, 95% CI 0.76, 0.81 at 1–7 days and over subsequent periods) and following a second dose of ChAdOx1 (IRR 0.84, 95% CI 0.82, 0.87 at 1–7 days; IRR 0.97, 95% CI 0.94, 0.99 at 8–14 days) and of BNT162b2 (IRR 0.85, 95% CI 0.83, 0.88 at 1–7 days; IRR 0.95, 95% CI 0.92, 0.98 at 8–15 days). There was an increased risk of cardiac arrhythmia following a second dose of mRNA-1273 (IRR 1.93, 95% CI 1.25, 2.96 at 1–7 days) and at 1–7 days (IRR 11.73, 95% CI 11.33, 12.14), 8–14 days (IRR 6.57, 95%CI 6.30, 6.85), 15–21 days (IRR 2.30, 95% CI 2.15, 2.45) and 21–28 days (IRR 1.67, 95% CI 1.55, 1.80) following a SARS-CoV-2 positive test.Over the 1–28 days post vaccination, we found a decreased risk of cardiac arrhythmia associated with a first dose of ChAdOx1 (IRR 0.94, 95% CI 0.93, 0.96) and BNT162b2 (IRR 0.89, 95% CI 0.87, 0.90) and following a second dose of ChAdOx1 (IRR 0.95, 95% CI 0.94, 0.96) and BNT162b2 (IRR 0.95, 95% CI 0.93, 0.96). There was an increased risk of cardiac arrhythmia following a second dose of mRNA-1273 (IRR 1.46, 95% CI, 1.08, 1.98) and a SARS-CoV-2 positive test (IRR 5.35, 95% CI 5.21, 5.50).Subgroup analyses by age group and sexTable 4 shows the IRRs for the outcomes in the overall 1–28 day risk periods before and after each exposure by sex and
https://www.nature.com/articles/s41591-021-01630-0
Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection