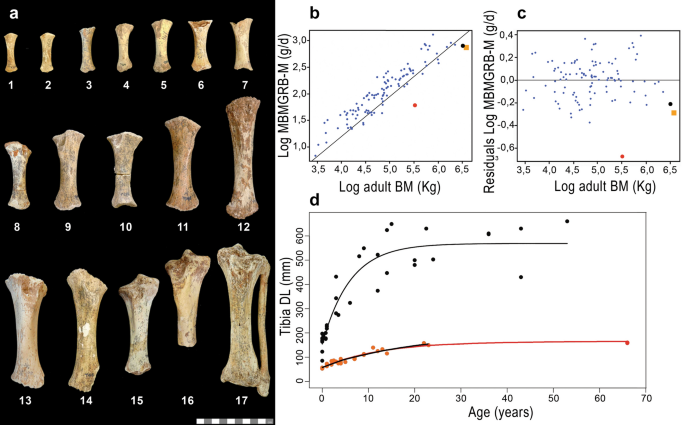
Palaeohistology reveals a slow pace of life for the dwarfed Sicilian elephant Meike Köhler1,2, Victoria Herridge3, Carmen Nacarino-Meneses1,4, Josep Fortuny1, Blanca Moncunill-Solé5,6, Antonietta Rosso7, Rossana Sanfilippo7, Maria Rita Palombo8 & Salvador Moyà-Solà1,2 Scientific Reports 11, Article number: 22862 (2021) Cite this article The 1-m-tall dwarf elephant Palaeoloxodon falconeri from the Pleistocene of Sicily (Italy) is an extreme example of insular dwarfism and epitomizes the Island Rule. Based on scaling of life-history (LH) traits with body mass, P. falconeri is widely considered to be ‘r-selected’ by truncation of the growth period, associated with an early onset of reproduction and an abbreviated lifespan. These conjectures are, however, at odds with predictions from LH models for adaptive shifts in body size on islands. To settle the LH strategy of P. falconeri, we used bone, molar, and tusk histology to infer growth rates, age at first reproduction, and longevity. Our results from all approaches are congruent and provide evidence that the insular dwarf elephant grew at very slow rates over an extended period; attained maturity at the age of 15 years; and had a minimum lifespan of 68 years. This surpasses not only the values predicted from body mass but even those of both its giant sister taxon (P. antiquus) and its large mainland cousin (L. africana). The suite of LH traits of P. falconeri is consistent with the LH data hitherto inferred for other dwarfed insular mammals. P. falconeri, thus, not only epitomizes the Island Rule but it can also be viewed as a paradigm of evolutionary change towards a slow LH that accompanies the process of dwarfing in insular mammals. Dwarfing, an adaptive evolutionary process1 by which the descendant grows to a smaller adult size than its ancestor, may evolve as a byproduct of selection acting primarily on LH characteristics, particularly on age at sexual maturity2 (ASM). LH theory posits that a small adult size can result from either advancement or postponement of sexual maturity, depending on the ecological scenario3,4,5. Broadly speaking, high extrinsic mortality (predation, parasite loads) curtails the time-period of growth (truncation) by forcing an early channeling of resources from growth to reproduction6, which results in growth arrest and an advanced onset of sexual maturity; the organism is small-at-maturity. Low resource availability, by contrast, forces a reduction in the rate of growth2,7, generally associated with prolonged allocation of resources to maintenance6,7 and a correlated delay in sexual maturity2,4,8; the organism remains small-for-age throughout ontogeny. Rate and duration of growth, hence, are the two variables (at least in determinate growers) that mediate the pace of life in response to prevailing environmental conditions.Dwarfing is a ubiquitous phenomenon that is particularly pervasive on islands where it affects large mammals and dinosaurs and, to a lesser extent, other vertebrates and even plants9,10,11,12,13. Insular dwarfing is now widely considered to be an adaptive response to selection pressures imposed by environmental conditions where net primary production and, hence, per capita food resources are low, and/or under elevated population density2,14,15,16,17,18,19,20,21,22, but see23,24,25,26. Paradoxically, despite the widely accepted notion that insular environments are characterized by low resource availability, the concept of growth truncation as the developmental process behind insular dwarfing is pervasive throughout literature23,24,25,26,27,28, even when growth rates (GRs) are slow27.Among herbivores, elephants have the slowest relative GRs29. Elephants form a group of very large mammals characterized by a slow LH with a slow GR, delayed age at maturity and long lifespan; indeed, they commonly live longer than 60 years in the wild30. Along with humans, they represent the slow end of the slow-fast LH continuum among terrestrial mammals, which is usually depicted as a mouse—elephant continuum. Through the scaling with body mass, their slow life-history is a direct result of their gigantic size. Unsurprisingly, hence, it is intuitively assumed that ‘dwarfed giants’ shifted towards a faster LH as in the case of P. falconeri23 from the late Middle Pleistocene of Sicily (Spinagallo cave, Syracuse, Supplementary material 1). At just 0.9 to 1.2 m tall, and with an estimated mean body mass of 252 kg (Supplementary material 2; Supplementary Tables 1, 2, 3), P. falconeri is the smallest elephant to have ever evolved; it weighted little more than 2% of its ancestor P. antiquus (11,500 kg31). Raia and colleagues23 calculated discrete LH values from interspecific scaling. Accordingly, the dwarf elephant attained sexual maturity at the age of 3–4 years, pregnancy took 189 days, and life span was 26 years. They considered a fast LH to be supported by an elevated number of unfused long bones interpreted as high calf mortality, and by the high number of tuskless females that supposedly arrested tusk growth to divert resources to reproduction. Because of their higher mass estimation for P. falconeri, Larramendi and Palombo25 provide similar but somewhat higher values. Roth21 suggested dwarfing in P. falconeri to be a consequence of selection for reduced energy use (through scaling of metabolic rate with body mass) and calculated a shortened growth period of 4 years based on body mass scaling. She furthermore speculated that by retention of a relatively long gestation period at a smaller body size, twins might have been more frequent than singletons. In current literature, P. falconeri is still being used as an example of evolution towards the fast end of the slow-fast LH continuum associated with insular dwarfing28.In this study, we reconstruct the key LH traits ‘age at maturity’ and ‘longevity’, as well as the rate and duration of growth in P. falconeri from a histological analysis of bones (skeletochronology; beginning of the external fundamental system EFS; accretional bone area), molars (daily enamel secretion rate ESR; enamel extension rate EER; plate formation time PFT; crown formation time CFT), and tusks (dentine daily secretion rate DSR; dentine extension rate DER; first FOI, second SOI, and third TOI order increments), using multiple technical (microscopy, 3D imaging) and statistical (von Bertalanffy; segmented regression) tools. We perform phylogenetic generalized least square regressions (PGLS) to evaluate potential phylogenetic signals in allometric life-history/body mass analyses of P. falconeri, extant ungulates and elephants. We rely on external measurements and 178 histological slides from 29 tibiae, 1 upper fourth deciduous tooth, 1 lower third molar, and 6 tusks. We compare the resulting LH data with those from the extant, full-sized continental cousin Loxodonta africana and, as far as available, with PFT of fossil continental Mammuthus columbi32 P. antiquus (own data), and insular P. cypriotes32, to establish whether P. falconeri dwarfed via growth truncation or GR reduction (see ‘Materials and methods’). In doing so, we aim to bring the first comprehensive data on insular dwarf elephant LHs to bear on the debate over causality between the process of dwarfing and the evolution of LH strategies on islands.To reconstruct growth pattern and LH schedule, an analysis of ontogenetic GRs in an allometric context is mandatory. Though elephants are known to have the slowest relative GRs29, the results of our analysis show that, despite its small size, P. falconeri grew at even slower relative rates than his continental cousins, with a peculiarly small difference in GRs between pre- and post-sexual maturity. We used different anatomical parts (tibiae, molars, and tusks) and different methodological approaches to infer GRs for P. falconeri. Thus, based on the ontogenetic growth of tibial diaphyses (Figs. 1a, 2; Supplementary material 3, 4; Supplementary Table 4; Supplementary Fig. 1), we found an extremely low scaling of the accumulated mean specific body mass GR from birth to maturity (MBMGRB-M), compared with other ungulates, including extant elephants (Fig. 1b, c; Supplementary material 3, 4). In accordance with the inferred slow juvenile GRs in P. falconeri, piecewise regression of the ontogenetic increase in length of the tibial diaphysis shows only minimal differences in the slopes of pre- and post-sexual maturity (5.77–2.45), which contrasts with the important differences in L. africana (40.62–5.77) (Fig. 3a; Table 1). Similar results have been obtained using von Bertalanffy growth models (Fig. 1d; Table 2). The value of the growth constant K in P. falconeri is far lower (0.055–0.079) than that of L. africana (0.1666) (see ‘Materials and methods’, Table 1). A similar pattern of GR is observable in the dentition. Tusk histology provided the most unexpected result of this study: the absence of relevant differences in GR over ontogeny (Figs. 4a, b, 5; Supplementary material 5). The overall tusk GR is even lower than in senescent extant Loxodonta, where it varies from 140 mm/y in young individuals to 20–40 mm/y in senescent females/males. Young individuals of P. falconeri, however, had a mean GR of 12.5 mm/y while the oldest grew at 10.4 mm/y (general sample mean 10.16 mm/y) (Fig. 4a, b; Supplementary material 5). Despite the statistically significant albeit very small differences between juvenile and adult tusk DSRs (Supplementary material 5, Supplementary Fig. 2, Supplementary Tables 8, 9), a trend towards uniformity in DER among tusks emerges from our analysis (Supplementary Figs. 3, 4, Supplementary Table 10). The scatterplot of log-transformed age (years) and log of extension rate (years) in P. falconeri (y = − 0.0446x + 1.0793, R2 = 0.22) shows the very low exponent, expressing the lack of significant differences in DER between young and old individuals (Fig. 4b). The minimal change in tusk GR over ontogeny parallels the small differences in postcranial GRs during pre- and post-sexual maturity (Supplementary material 6; Figs. 1d, 3a). A similarly slow GR is observable in molars (Figs. 6c, 7). Plotted against PFT, the molar plates grew consistently slower in height in P. falconeri (and in P. cypriotes) than in continental elephants (Mammutus columbi, P. antiquus) (Fig. 6c; Supplementary material 7). Accordingly, insular dwarf taxa combine a slower rate of dental growth (plate height against PFT) with an unchanged time of formation. When postcranial (tibiae), tusks and molars are considered together, a pattern of systemic slow GRs emerges for P. falconeri compared with the element- and age-dependent variable GRs in L. africana.Figure 1P. falconeri GR. (a) sample of the aged tibiae: 1,2: neonates (CAT-106; CAT-108); 3: one year (ELEPH1-T); 4: two years (CAT-124); 5: 3,5 years (CAT-128); 6,7,8: 4 years (CAT-132, Cat 133, ELEPH2-T); 9,10: 7 years (CAT 139, Cat 140); 11,12: 9 years (CAT-45, 142); 13: 12 years (ELEPH3-T); 14: 13 years (CAT-46); 15: 14 years (CAT-179); 16: 18 years (T-181);17: 23 years (Roma 6) (Graphic scale 10 cm). (b) Scaling of mean body mass GR from birth to maturity (MBMGR, expressed in grams/day) in respect to adult body mass, in log10, using phylogenetic generalized least square regressions (PGLS), in a large sample (n = 106) of species of ungulates (see Supplementary material 8 for results of PGLS). Blue dots: ungulates; red dots: P. falconeri (body mass from tibia LTC; Supplementary material 2; Supplementary Tables 2, 3), black dot: Elephas maximus; orange square: L. africana; data from PanTheria33. (c) distribution of residuals of the PGLS log10MBMGRB-M (adult-neonatal body mass, gr/age from birth in days) (y) regressed against log10 adult body mass (x). Extant elephants (orange square: L. africana, black dot: E. maximus) are within the lower limit of adult body mass-neonatal body mass GR in ungulates; P. falconeri (red dot) corresponds to the body mass estimation of tibia LTC (Supplementary material 2). (d) Von Bertalanffy growth curves of diaphyseal lengths of the tibiae (TDL) of L. africana (black dots and black curve) and P. falconeri (red dots; black and red curves). P. falconeri growth model 1 (short black curve) is based on ontogenetic data of specimens directly aged by skeletochronology (red dots). The long red curve corresponds to model 2 and is based on the inferred minimal longevity (Table 1; ‘Materials and methods’) and the maximal value of TDL.Figure 2Tibia midshaft sections of P. falconeri. Representation of tissue types in histological sections of an ontogenetic series of tibiae ((a, b, f, e, I, g, k); anterior crest upper); magnifications (c, d, h, j; medullary cavity upper). (a) neonate (T106) showing parts of the birth line; (b, c) (T1): 1 year; longitudinal and radial osteons after the first winter LAG; (d) (T135), (e) (T138): 2.5–3 years ‘Fibro-lamellar complex’ (FLC) with longitudinal, oblique and radial osteons; (f) (T2): 4 years; (g) (T139): 7 years; (h) (T 45): 9 years, still showing active growth (open canals at the outer cortex); (i) (T142): 11 years; (j) (T 203): 22 years, showing an EFS; (k) (Roma6): 23 years;. Zeiss Scope A1 microscope with integrated digital camera (AxioCam ICc5), transmitted light. 10 mm scale: complete sections; 2000 µm scale: magnifications.Figure 3Inference of ASM in P. falconeri. (a) Piecewise regressions of tibia diaphyseal lengths (DL) against age (years) in L. africana and P. falconeri. Open black circles and black regression line represent L. africana; large black dot: break point (BP) with its value; open red circles and red regression line correspond to P. falconeri; large red dot: break point (BP) with its value; slopes of regressions are placed over the lines for each taxon (Table 2). (b) Ontogeny of the reproductive phases of L. africana, shown as age-specific conception rates and reproductive activity for a sample of 905 L. africana cows shot in the Krüger National Park (South Africa) between 1970 and 197434, signaling the reproductive ontogenetic phases. Horizontal bar in the upper left corner indicates the timing of the break point (BP) obtained from DL of the tibia with the 95% confidence intervals (See ‘Materials and methods’; Supplementary material 6). (c) ASM: scatterplot of phylogenetic generalized least square regressions (PGLS) of log ASM (y) against body mass (x) for extant ungulates (n = 145) and elephants (Data base from AnAge35). Results of PGLS regression in Supplementary material 8. Blue dots: ungulates; red dot: P. falconeri; green dot: Myotragus balearicus36; black dot: Elephas maximus; orange square: L. africana; (d) Residuals of ASM in ungulates from PGLS regression of ASM against body mass. Blue dots: ungulates; Red dot: P. falconeri; Green dot: Myotragus balearicus36; Black dot: Elephas maximus; orange square: Loxodonta africana (Data from AnAge Data base35).Table 1 Statistical results from von Bertalanffy growth model for diaphyseal length of the tibia (DL) of P. falconeri and L. africana.Table 2 Piecewise regressions of tibia diaphyseal lengths of L. africana and P. falconeri.Figure 4Tusk growth patterns of P. falconeri. (a) Spinagallo tusks sample analysed. 1: CAT-5; 2: CAT-24; 3: CAT-51; 4: CAT-100; 5: CAT-102; 6: CAT-77-76-78. Graphic scale 5 cm. (Supplementary material 5, Supplementary Table 5). (b) changes in ER over ontogeny (log-transformed age (x) and log of ER (y)) in L. africana37 and P. falconeri. Blue dots: L. africana females (y = − 0.5116x + 2.291, R2 = 0.88); orange dots: L. africana males (y = − 0.3402x + 2.211
https://www.nature.com/articles/s41598-021-02192-4
Palaeohistology reveals a slow pace of life for the dwarfed Sicilian elephant