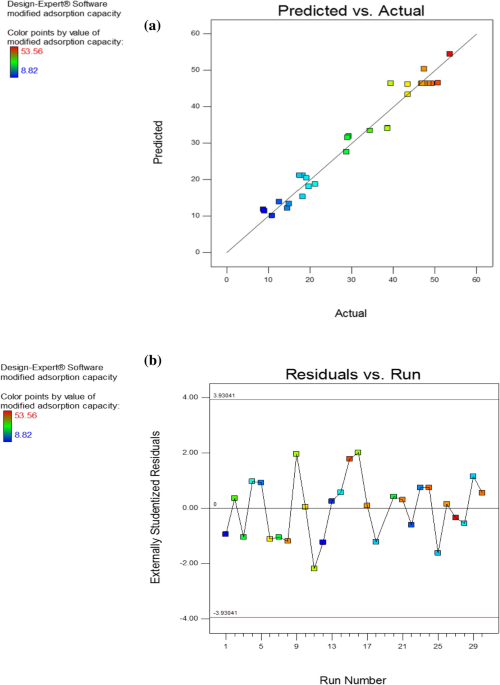
Optimized production, Pb(II) adsorption and characterization of alkali modified hydrochar from sugarcane bagasse Today, sugarcane bagasse (SB) is used for bioethanol and biodiesel production, energy generation, and adsorbent synthesis. The goal of this project is to determine the optimized conditions for producing adsorbent from sugarcane bagasse using hydrothermal carbonization (HTC) and KOH activation. To optimize process parameters such as reaction temperature, residence time, ZnCl2/SB mixing ratios, and water/SB mixing ratios, response surface methodology was used. The results revealed that the optimum modified adsorption occurred at 180 °C, 11.5 h, a water to biomass ratio of (5:1), and a ZnCl2 to precursor ratio of (3.5:1). The physicochemical features of optimum activated hydrochar were investigated, as well as batch adsorption experiments. The pseudo-second-order kinetic model and the Langmuir isotherm model were found to fit the experimental results in batch adsorption studies [({q}_{max}=90.1) (mg/g)]. Thermodynamic experiments further confirmed the spontaneous and exothermic adsorption mechanism. Agricultural biomass waste is made up of organic substances created by humans in agricultural activities. This waste can be used as a raw material for the manufacturing of valuable products such as fuels, biogas, and adsorbents due to its abundance and availability1,2. Sugarcane bagasse (SB) is a cellulose, hemicellulose, and lignin-rich agricultural biomass waste. In the past, SB was used for energy generation, but it is now a great substrate for environmentally friendly approaches like bioethanol, energy generation, biodiesel, and adsorbent synthesis3. SB can be a suitable candidate for adsorbent production and removing pollutants such as lead from the environment.Lead is one of the most common and dangerous metals found in smelting, battery, and coating industry effluents. Pb2+ can enter the body through the skin, digestive tract, and respiratory tract, causing damage to people (brain, mental, renal, and liver illness, anemia, and other disorders), and will accumulate in mammal bodies, according to research. Chemical precipitation, ion exchange, liquid membrane, electrochemical, and adsorption procedures are among the various ways utilized to remediate lead-polluted wastewater today. Adsorption is a good alternative for heavy metal handling because of its basic equipment, ease of operation, low cost, and acceptable efficiency4. Today hydrothermal carbonization is used as a green technology for biomass waste management to create valuable materials such as adsorbents.Hydrothermal carbonization (HTC) is a primarily exothermic thermochemical conversion process that converts waste biomass into useful materials at a low temperature (180–250 °C) and under autogenous pressure. HTC’s products include hydrochar (HC), a carbonaceous coal-like solid material with rich oxygen functional groups, process water (a mixture of bio-oil and water), and a small amount of gases5,6. The type of waste biomass used, as well as process parameters such as temperature, resident time, water to biomass ratio, catalyst quantity, and activation method, all influence the characteristics of the HC generated7. Water works as a solvent medium, reactant, and catalyst in the HTC process because to the changing physicochemical properties of water at elevated temperatures (ionization strength and dielectric constant). It contributes to waste biomass hydrolysis and cleavage7,8.The raw material undergoes numerous important reactions during the HTC process. The initial step in each reaction is hydrolysis. After that, a series of reactions will be carried out, including dehydration, decarboxylation, condensation, and polymerization9,10. HC can be used as a fuel, soil amelioration, and a desirable material for adsorbent material. Because HC has a lower oxygen to hydrogen and hydrogen to carbon ratio (O/H and H/C) and higher oxygenated functional groups (OFGs) than raw material, it can be used as a suitable candidate for production of activated carbons and adsorbents11,12.Activated carbons play an important role in wastewater treatment due to their ease of handling, low processing costs, and higher adsorption effectiveness than other pollutant removal methods13,14. HTC of lignocellulosic biomass for the removal of organic and inorganic pollutants from wastewater is a promising technology due to the low cost of feedstock materials, the use of renewable and diverse sources of material, and the ecologically beneficial process.In recent years, the generation of activated carbon from HTC of biomass such as Cotton stalk15, avocado seed16, flax shives, and oat hulls17, mango peels18, palm leaves19, cassava slag1, brewer’s spent grain20, corncob21, rice straw22, Lepironia articulate23, banana fruit bunch24, hickory wood and peanut hull25, bamboo shoot26, Teak Sawdust27, bamboo sawdust28, Agave americana fibres and mimosa tannin29, prolifera-green-tide30 have been studied.To the best of knowledge of the authors, no study has looked into the complete impact of process factors (temperature, resident time, biomass to water ratio, and biomass to catalyst ratio) on the adsorption capacity of waste biomass-derived hydrochar. The effect of process factors on the HTC of sugarcane bagasse with alkali alteration was investigated in this experiment, and optimum adsorption conditions were discovered. After then, the properties of activated hydrochar were investigated. The main purpose of this study is to look into the optimized process parameters for HTC of BG based on adsorption capacity and hydrochar yields for the first time.The study’s precise objectives were to: (a) Assess the potential of HTC of SB for lead adsorption. (b) Using a central composite design-response surface methodology (CCD-RSM) technique, investigate the effect of process variables (temperature, resident time, water to biomass ratio, and catalyst dose) on changed adsorption capacity. (c) Performing batch adsorption study to investigate isotherm, kinetic and thermodynamic study, and characterization of optimum adsorbent. Table 1 lists the independent factors and response values for each experiment. The CCD design was arranged into 30 runs with 4 variables and five levels, 16 full factorial design matchings, 8 axial trials, and 6 repeats in the central position. The modified adsorption capacity (MAC) of activated hydrochar (AHC) increases from 6.70 ± 0.42 to 53.56 ± 1.98 (mg/g), indicating that HTC parameters had a significant impact on adsorption capacity and yield.Table 1 Independent variables and corresponding response value.Model fitting and analysis of variance (ANOVA)The hypothesis testing (p-value) and F-test (Fisher test) were used to evaluate significant factors and model fitting. Table S1 (Supplementary information) shows the results of the analysis of variance (ANOVA) for the independent variables temperature (A), water to biomass ratio (B), ZnCl2 to biomass ratio (C), and resident time (D). The linear terms A, B, C, and D, as well as the quadratic terms A2, C2, D2, and the synergy term AD, were noteworthy variables. Equation (1) describes the second-order regression equation adjusted to the response (MAC) in the form of coded values after eliminating non-important variables.$$MAC=46.38-8.81A-4.06B+3.73C+5.55D-12.66{A}^{2}-1.83{C}^{2}-5.86{D}^{2}-4.63AD$$Temperature and resident time were more significant than other factors according to the fitting equation and F-test (Table S1). The most important variable in changed adsorption capacity was temperature (F-value equal 83.80). The temperature and resident time interaction was the most important of the various interactions between the variables (Table S1).Table S2 shows the ANOVA for the response model. The response model has a reasonable F-value of 27.04 and a p-value of 0.0001 (less than 0.05), as well as a lack of fit p-value of 0.37 (higher than 0.05)31.Figure 1 depicted the scattering of residues as well as the link between actual (experimental) and predicted values (Fig. 1a,b). As can be seen in Fig. 1a, the near proximity of the points to the predefined line suggested that the regression model’s actual and anticipated values were quite close. As shown in Fig. 1b, with a random distribution and low residue values, the model is substantial32,33.Figure 1Relationship between the actual (experimental) and predicted values (a) and plot of the residues versus experimental order (b).Response of surfacesThe effects of two-factor interactions (the other variables fixing at coded value of zero) on the MAC were investigated, and the findings are shown in Fig. 2. As shown in Table S1, regarding F-value, temperature is the most important variable, and interactions between temperature and other factors are more relevant than other conceivable interactions. Furthermore, temperature and time played a crucial role in adsorption performance. So the effect of time and temperature (as fixed parameters) on two-factor interactions were represented in Fig. S1. The quantity of oxygenated functional groups (OFGs) in hydrochar based adsorbent influences its adsorption capability. Previous study showed that temperature and resident time influenced the amount of OFGs in HC7. The response of the surfaces for synergy terms (AB, AC, …, CD) are shown in Fig. 2. Furthermore, other variables are fixed at zero coded value, and the effect of temperature and resident time on the synergy coefficients is shown in Fig. S1.Figure 2Three-dimensional response surface for MAC: water to biomass ratio versus temperature (a), temperature versus ZnCl2 to biomass ratio (b), temperature to time ratio (c), water to biomass ratio versus ZnCl2 to biomass ratio (d), water to biomass ratio versus time (e) and time to ZnCl2 to biomass ratio (f).The interplay of temperature with the water-to-biomass ratio (other two variable fixed at zero coded value) is depicted in Fig. 2a. The MAC increased and then dropped with rising temperature at any water to biomass ratio. It’s possible that the oxygenated functional groups grew as the temperature rose and subsequently reduced at a higher temperature34,35. As shown in Fig. S1, the interaction between temperature and water-to-biomass ratio at different times are similar but at a lower resident time, maximum MAC occurred at the higher temperature. The connection between temperature and the ZnCl2 to biomass ratio (other two variable fixed at zero coded value) is seen in Fig. 2b. At any ZnCl2 to biomass ratio, with rising temperature, the modified adsorption capacity grew, reached its maximum, and then decreased, similar to the previous interaction. As shown in Fig. S1, the interaction between temperature and ZnCl2 to biomass ratio are similar at different times, but at a higher resident time, maximum MAC occurred at the lower temperature. The temperature and resident time interaction (other two variable fixed at zero coded value) is represented by Fig. 2c. Similar to the preceding interactions, the maximum adsorption was obtained with increasing temperature at any time, and thereafter it declined36. Unlike earlier interactions, time dependency of response decreased as temperature increased, therefore time became more important at lower temperatures. It could be explained as the dependency of OFGs on temperature and time. At a specific temperature, with rising times, the OFGs reached a maximum and then decreased. At higher temperatures, the rate of reactions in the HTC process increased, and maximum OFGs formation and time to reach equilibrium OFGs decreased7. The relationship between the water to biomass ratio and the ZnCl2 to biomass ratio (other two variable fixed at zero coded value) is shown in Fig. 2d. Compared to other interactions, this one had fewer consequences. The adsorption rose marginally as the amount of ZnCl2 to biomass ratio was raised while the amount of water to biomass ratio was decreased. The interaction between the water to biomass ratio and the ZnCl2 to biomass ratio at different times and temperatures are shown in Fig. S1. The trend of MAC changing concerning the water to biomass ratio and the ZnCl2 to biomass ratio is similar regardless of time and temperature. At higher temperatures, the dependency of MAC on temperature is reduced, but at a lower temperature, with rising time, the MAC is raised. The interplay between time and the water-to-biomass ratio (other two variable fixed at zero coded value) is depicted in Fig. 2e. The adsorption reduced as the water content increased, but at any water to biomass ratio, the reaction reached a maximum and then decreased marginally as time passed. The interaction between the water to biomass ratio and the time at different temperatures is shown in Fig. S1. The trend of MAC changing concerning the water to biomass ratio and time is similar, But with rising temperature, the maximum MAC occurs at a lower resident time. The interplay between time and the ZnCl2 to biomass ratio (other two variable fixed at zero coded value) is depicted in Fig. 2f. With increasing time, the adsorption of any ZnCl2 ratio increased, then decreased slightly. The interaction between time and the ZnCl2 to biomass ratio at different temperatures is shown in Fig. S1. With rising temperature, the maximum MAC occurs at a lower resident time. At any ZnCl2 to biomass ratio, with increasing time, the OFGs formed and reached the maximum. Then they decreased because of transformation OFGs to stable oxygen surface groups by excessive dehydration/carbonization reaction or breakdown of OFGs to the gaseous product7.OptimizationSoftware delivered 100 optimization conditions across a range of experimental research. Economic consideration depicted that the lower temperature is preferable to the higher temperature among the various optimization conditions that maximize responses (MAC).The optimum temperature is 180 °C, with a water-to-biomass ratio of 5 (w/w), a ZnCl2-to-biomass ratio of 3.5 (w/w), and a resident time of 11.5 h.
https://www.nature.com/articles/s41598-021-01825-y
Optimized production, Pb(II) adsorption and characterization of alkali modified hydrochar from sugarcane bagasse