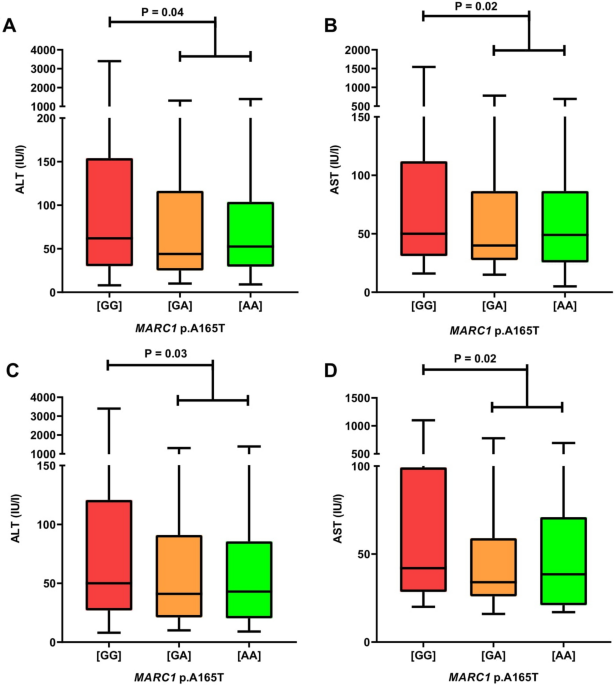
MARC1 p.A165T variant is associated with decreased markers of liver injury and enhanced antioxidant capacity in autoimmune hepatitis Maciej K. Janik1,2, Wiktor Smyk1,2, Beata Kruk3, Benedykt Szczepankiewicz4, Barbara Górnicka4, Magdalena Lebiedzińska-Arciszewska5, Yaiza Potes5, Inês C. M. Simões5, Susanne N. Weber6, Frank Lammert6,7, Mariusz R. Więckowski5, Piotr Milkiewicz1,2,8 & Marcin Krawczyk3,6 Scientific Reports 11, Article number: 24407 (2021) Cite this article The clinical picture of autoimmune hepatitis (AIH) varies markedly between patients, potentially due to genetic modifiers. The aim of this study was to evaluate genetic variants previously associated with fatty liver as potential modulators of the AIH phenotype. The study cohort comprised 313 non-transplanted adults with AIH. In all patients, the MARC1 (rs2642438), HSD17B13 (rs72613567), PNPLA3 (rs738409), TM6SF2 (rs58542926), and MBOAT7 (rs641738) variants were genotyped using TaqMan assays. Mitochondrial damage markers in serum were analyzed in relation to the MARC1 variant. Carriers of the protective MARC1 allele had lower ALT and AST (both P < 0.05). In patients treated for AIH for ≥ 6 months, MARC1 correlated with reduced AST, ALP, GGT (all P ≤ 0.01), and lower APRI (P = 0.02). Patients carrying the protective MARC1 genotype had higher total antioxidant activity (P < 0.01) and catalase levels (P = 0.02) in serum. The PNPLA3 risk variant was associated with higher MELD (P = 0.02) in treated patients, whereas MBOAT7 increased the odds for liver cancer (OR = 3.71). None of the variants modulated the risk of death or transplantation. In conclusion, the MARC1 polymorphism has protective effects in AIH. Genotyping of MARC1, PNPLA3, and MBOAT7 polymorphisms might help to stratify patients with AIH. Autoimmune hepatitis (AIH) is a progressive liver disease, which, if untreated, leads to liver cirrhosis1. Therapy for AIH is based on immunosuppression, which induces remission in the majority of patients; some patients may require lifelong maintenance therapy or treatment with alternative, second-line drugs1. The progression of AIH varies markedly between patients2, and up to 30% of patients have liver cirrhosis at the time of diagnosis.In the last decade, several inherited determinants of liver injury were identified3. For example, the common PNPLA3 p.I148M, MBOAT7 p.G17E, and TM6SF2 p.E167K variants have been shown to increase the risk of liver steatosis, fibrosis, and cirrhosis in patients with nonalcoholic fatty liver disease (NAFLD), alcoholic liver disease (ALD), and with viral liver diseases3,4,5,6. Recently, two variants, HSD17B13 rs72613567:TA7 and MARC1 p.A165T8,9,10, were shown to decrease the risk of developing NAFLD. The MARC1 p.A165T variant was demonstrated to have protective effects in patients with alcoholic liver disease (ALD)11. Rare liver diseases, such as AIH, have not been thoroughly studied in terms of genetic modifiers of their progression. A genome-wide association study (GWAS) in 649 adults with AIH from the Netherlands identified human leukocyte antigen (HLA) loci, HLA-DRB1*0301 and HLA-DRB1*0401, as susceptibility genotypes12, which was in line with other genetic studies on AIH13,14,15. The strongest non-HLA susceptibility marker was found to be SH2B312, which was also reported to increase the risk of developing other autoimmune diseases, such as primary sclerosing cholangitis (PSC)16, primary biliary cholangitis (PBC)17, and type 1 diabetes mellitus18. Analyses of PBC and PSC risk loci in patients with AIH without cholestatic variants revealed that part of the genetic susceptibility for AIH coincides with PBC and PSC12. Other non-HLA susceptibility polymorphisms, for example, tumor necrosis factor alfa (TNF-alpha)19 or vitamin D receptor (VDR)20,21, were also associated with AIH in the previous studies. A recently published single-center analysis of 239 adult patients with AIH demonstrated that carriers of the minor PNPLA3 p.I148M allele are characterized by advanced liver fibrosis according to the AST to Platelet Ratio Index (APRI) and Fibrosis-4 Index (FIB-4)22. Furthermore, it was shown that the PNPLA3 p.148MM genotype might increase the odds of liver transplantation or death in patients with AIH22; however, these results have not yet been validated in other cohorts of patients with AIH.In our current study, we investigate the role of inherited predisposition in the progression of liver injury in patients with AIH. To this end, we genotyped PNPLA3, MBOAT7, TM6SF2, HSD17B13, and MARC1 variants in a large cohort of adult patients with AIH and performed the genotype–phenotype analyses.Between October 2015 and June 2019, we prospectively enrolled 313 consecutive non-transplanted adults with AIH at the Medical University of Warsaw, Poland. Pure AIH, as well as its cholestatic variants, namely AIH-PSC and AIH-PBC, were diagnosed according to the current guidlines1 (i.e., they were based on liver histology, presence of typical antibodies, elevated serum concentration of immunoglobulin G, and, in cholestatic variants, imaging studies). The diagnosis of AIH-PSC overlap was based on imaging or histological features of PSC combined with features (biochemical, serological, and histological) of AIH. AIH-PBC diagnosis was established according to the 'Paris criteria'23. Any acute or chronic liver diseases other than AIH, or its overlap with PSC and PBC, served as exclusion criteria.The study protocol (KB/128/2015) was approved by the Ethics Committee of the Medical University of Warsaw, according to the ethical guidelines of the 1975 Declaration of Helsinki (latest revision, 2013), and written informed consent was obtained from all participants.Clinical dataAll patients underwent careful clinical examination. Blood samples were drawn from fasted subjects. Non-invasive measurements for liver fibrosis and blood tests were performed in all patients on the same day, i.e., upon enrolment in the study. Liver fibrosis was non-invasively quantified using real-time 2D-SWE by Aixplorer (SuperSonic Imagine, Aix-en-Provence, France), as described previously24, and is presented as LSM. The following serum fibrosis indices were calculated as follows: FIB-4 was calculated according to Sterling et al.25, and the APRI was calculated in line with Wai et al.26. Liver biopsies were available in 48 (80%) of therapy-naïve patients; LB was not performed in 12 (20%) of therapy-naïve patients due to ascites, coagulopathy, or lack of patient consent. Biopsies were analyzed by a pathologist experienced in liver diseases using the Batts and Ludwig27 system for the assessment of liver fibrosis and inflammation. The available clinical data from the follow-up were used to examine changes in blood tests in relation to the studied polymorphisms.Given the differences between pure AIH and its cholestatic variants, we performed genotype–phenotype association tests in the entire cohort of patients and separately in subsets of patients with pure AIH and with AIH-PSC or AIH-PBC. The subgroup of patients with pure AIH was divided in relation to the immunosuppressive therapy (i.e., therapy ≥ 6 months or therapy-naïve).Data at diagnosis, clinical outcome, and hepatocellular carcinomaIn addition to the data collected at baseline, we analyzed the association between the studied polymorphisms and (1) age and presence of liver cirrhosis at diagnosis, as well as the type of AIH; (2) clinical outcome defined as a combined endpoint: liver transplantation (LT) or liver-related death; and (3) hepatocellular carcinoma (HCC). Due to the relatively small number of patients who reached the clinical endpoint in the study group (n = 66, 21%), the additional cohort of 30 patients after LT for AIH (57% female, median age at diagnosis 33 (range 8–62) years, median age at LT 35 (range 12–67) years) was included in this analysis. The diagnosis of HCC was established by imaging studies (CT or MRI) and confirmed by histology, if available.GenotypingGenotyping of the MARC1 (rs2642438), PNPLA3 (rs738409), TM6SF2 (rs58542926), MBOAT7 (rs641738), and HSD17B13 (rs72613567) gene variants was performed at Saarland University Medical Center in Homburg, Germany, by technicians blinded to the phenotype of patients. A detailed description of genotyping is provided in Supplementary Text S1 and Table S1.Hepatocyte mitochondrial damage in relation to the MARC1 genotypeHow MARC1 may impact liver damage is unclear; however, this signal-anchored mitochondrial protein28 is associated with detoxification reactions29. Thus, we evaluated the presence of oxidative stress-induced mitochondrial damage markers of hepatocytes in serum. The level of mitochondrial uncoupling protein 2 (UCP2) (an inner mitochondrial membrane marker) and two antioxidant enzymes, thioredoxin reductase 2 (TrxRd2) and superoxide dismutase 2 (SOD2) (mitochondrial matrix markers), in the serum of 39 individuals carrying either the wild-type (n = 25) or the homozygous (n = 14) MARC1 variant were evaluated. This cohort was randomly selected from the pure AIH group (without AIH overlap with PSC and PBC) treated for at least 6 months. Patients for this analysis were randomly selected from the AIH cohort using SPSS software (IBM Corp. Released 2020. IBM SPSS Statistics for Windows, Version 27.0. Armonk, NY: IBM Corp.).Western blot analysisThe presence of antioxidant enzymes: ubiquitous catalase (CAT) and mitochondrially located TrxRd2 and SOD2, or mitochondrial proteins like UCP2 in serum, which could be linked to damage of hepatocytes, was evaluated with the use of specific antibodies, as described in detail in Supplementary Text S2.Determination of oxidative damage markers in serumLipid peroxidation (LPO) in serum was evaluated by measuring malondialdehyde (MDA) (a major reactive aldehyde resulting from the peroxidation of biological membranes) using the Lipid Peroxidation Assay Kit from Abcam (ab118970, Cambridge, U.K.). Serum protein oxidative damage was estimated using OxyBlot Protein Oxidation Detection Kit (S7150, Sigma-Aldrich, MO, USA). A detailed description of the performed experiments is provided in Supplementary Text S2.Serum total antioxidant activityThe concentration of small molecule antioxidants and small protein antioxidants in serum samples was analyzed by measuring the total antioxidant activity (TAA) following the ABTS/H2O2/HRP method developed by Arnao30 with modifications by Gonzalo-Calvo31 (Supplementary Text S2).Statistical analysesStatistical analyses were performed using SPSS (IBM Corp. Released 2020. IBM SPSS Statistics for Windows, Version 27.0. Armonk, NY: IBM Corp.) and GraphPad Prism (GraphPad Prism version 8.0.0 for Windows, GraphPad Software, San Diego, California USA, http://www.graphpad.com). The consistency of genotyping results with the Hardy–Weinberg equilibrium (HWE) was tested by exact tests (https://ihg.gsf.de/cgi-bin/hw/hwa1.pl). A two-sided P-value < 0.05 was considered statistically significant. Kolmogorov–Smirnov or Shapiro–Wilk tests were used to determine whether the set of observations followed normal distributions. Student's t and Mann–Whitney U tests were used to study normally and non-normally distributed parameters, respectively. Analyses of variance (ANOVA) and Kruskal–Wallis tests were applied to study differences between three groups with normally and non-normally distributed parameters, respectively. The Wilcoxon signed-rank test was used to compare paired blood test results from baseline and follow-up. The associations between gene variants and follow-up data were tested by logistic regression analyses. Principal component analysis (PCA) was performed with R (2020, R Core Team, Vienna, Austria). The impact of the tested polymorphisms on the clinical endpoint (defined as LT or liver-related death), as well as diagnosis of HCC, was estimated with Cox regression and Kaplan–Meier survival analysis.Clinical details of the study cohort are presented in Table 1. In total, 313 adults with AIH (70% women) were recruited; their median age was 36 (range 18–83) years. Pure AIH, AIH-PSC, and AIH-PBC were diagnosed in 206 (66%), 77 (25%), and 30 (9%) recruited patients, respectively. The median duration of the disease was 4 (range 0–33) years. In total, 60 (19%) patients were therapy naïve at inclusion. Liver cirrhosis, diagnosed by LB or LSM and by clinical signs of decompensation, was present in 130 (42%) patients at the enrolment in the study. Almost 70% of the entire cohort received steroid-based therapy, which is summarized in Table 1. Almost all patients with cholestatic variants were treated with ursodeoxycholic acid. Approximately 40% of patients were on steroid-based therapy after 2 years of therapy, whereas more than 20% of cases achieved long-term biochemical remission (more than 2 years) and were not receiving immunosuppression at the baseline.Table 1 Clinical and biochemical data of the study cohort. MARC1 shows protective effects in patients with AIHThe five genetic variants were successfully genotyped in all patients. Table 2 presents the distributions of genotypes. All variants were within the Hardy–Weinberg equilibrium, underscoring robust genotyping.Table 2 Genotype frequencies in the study group.The MARC1 polymorphism showed prominent modulating effects on the AIH phenotype. In the entire cohort of 313 individuals, carriers of the MARC1 minor allele had significantly lower serum activities of ALT (P = 0.04) and AST (P = 0.02) (Fig. 1A,B) at baseline. Comparable results were found in patients with pure AIH (n = 206); carriers of at least one minor allele had significantly lower serum ALT (P = 0.03) and AST activities (P = 0.02) (Fig. 1C,D).Figure 1MARC1 minor allele is associated with lower serum ALT and AST activities in patients with AIH (A,B results in the entire study cohort, n = 313; C,D results in patients with pure AIH (i.e. without cholestatic overlaps), n = 206). [GG], MARC1 wild-type; [GA], MARC1 heterozygous variant; [AA], MARC1 homozygous variant; ALT, serum alanine aminotransferase level; AST, aspartate aminotransferase; MARC1, mitochondrial amidoxime-reducing component 1.Figure 2 shows the results of 146 patients with pure AIH who were treated for ≥ 6 months. In these patients, the frequency of individuals with normal ALT increased with the number of protective MARC1 alleles (Fig. 2A). As shown in Fig. 2B, carriers of the protective MARC1 allele had significantly lower serum AST activities (P = 0.01), and homozygous carriers of the protective allele presented with lower ALP (P < 0.01, Fig. 2C) and GGT activities (P < 0.01, Fig. 2D) as compared to patients with the wild-type genotype. Carriers of the MARC1 minor allele also had lower APRI (median 0.61, range 0.15–7.28) compared to the carriers of the wild-type genotype (median 0.81, range 0.18–14.04; P = 0.02). Finally, the MARC1 p.A165T polymorphism was associated with lower immunoglobulin G (IgG) concentrations (P 1600 mg/dL; n = 53). LSM, MELD, and liver histology were not modulated by this polymorphism.Figure 2MARC1 variant and clinical data in patients treated for at least 6 months for AIH without cholestatic overlaps (n = 146; A distribution of patients with normal or increased ALT in relation to the MARC1 genotype; B-D effects of this variant on serum AST, ALP and GGT). [GG], MARC1 wild-type; [GA], MARC1 heterozygous variant; [AA], MARC1 homozygous variant; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transferase; UNL, upper normal limit; MARC1, mitochondrial amidoxime-reducing component
https://www.nature.com/articles/s41598-021-03521-3
MARC1 p.A165T variant is associated with decreased markers of liver injury and enhanced antioxidant capacity in autoimmune hepatitis