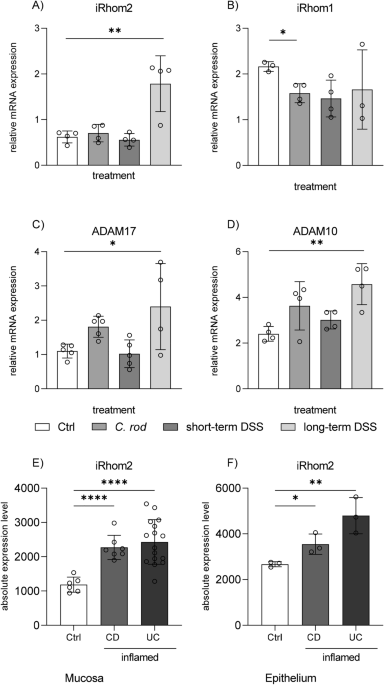
1.Abdulkhaleq, L. A. et al. The crucial roles of inflammatory mediators in inflammation: A review. Vet World https://doi.org/10.14202/vetworld.2018.627-635 (2018).Article PubMed PubMed Central Google Scholar 2.Ariel, A. & Timor, O. Hanging in the balance: Endogenous anti-inflammatory mechanisms in tissue repair and fibrosis. J. Pathol. https://doi.org/10.1002/path.4108 (2013).Article PubMed Google Scholar 3.Eming, S. A., Krieg, T. & Davidson, J. M. Inflammation in wound repair: Molecular and cellular mechanisms. J. Invest. Dermatol. https://doi.org/10.1038/sj.jid.5700701 (2007).Article PubMed Google Scholar 4.Barrientos, S., Stojadinovic, O., Golinko, M. S., Brem, H. & Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. https://doi.org/10.1111/j.1524-475X.2008.00410.x (2008).Article PubMed Google Scholar 5.Peschon, J. J. et al. An essential role for ectodomain shedding in mammalian development. Science (80-). https://doi.org/10.1126/science.282.5392.1281 (1998).Article Google Scholar 6.Schulz, B. et al. ADAMIO regulates endothelial permeability and T-cell transmigration by proteolysis of vascular endothelial cadherin. Circ Res. https://doi.org/10.1161/CIRCRESAHA.107.169805 (2008).Article PubMed PubMed Central Google Scholar 7.Hartmann, D. et al. The disintegrin/metalloprotease ADAM 10 is essential for Notch signalling but not for α-secretase activity in fibroblasts. Hum. Mol Genet. https://doi.org/10.1093/hmg/11.21.2615 (2002).Article PubMed Google Scholar 8.Koenen, R. R. et al. Regulated release and functional modulation of junctional adhesion molecule A by disintegrin metalloproteinases. Blood https://doi.org/10.1182/blood-2008-04-152330 (2009).Article PubMed Google Scholar 9.Lee, D. C. et al. TACE/ADAM17 processing of EGFR ligands indicates a role as a physiological convertase. Ann. New York Acad. Sci. https://doi.org/10.1111/j.1749-6632.2003.tb03207.x (2003).Article Google Scholar 10.Pruessmeyer, J. et al. A Disintegrin and metalloproteinase 17 (ADAM17) mediates inflammation-induced shedding of syndecan-1 and -4 by lung epithelial cells. J Biol Chem. https://doi.org/10.1074/jbc.M109.059394 (2010).Article PubMed Google Scholar 11.Black, R. A. et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-∅ from cells. Nature https://doi.org/10.1038/385729a0 (1997).Article PubMed Google Scholar 12.Moss, M. L. et al. Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-α. Nature https://doi.org/10.1038/385733a0 (1997).Article PubMed Google Scholar 13.Rosendahl, M. S. et al. Identification and characterization of a pro-tumor necrosis factor-α- processing enzyme from the ADAM family of zinc metalloproteases. J. Biol. Chem. https://doi.org/10.1074/jbc.272.39.24588 (1997).Article PubMed Google Scholar 14.Sahin, U. et al. Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J. Cell Biol. https://doi.org/10.1083/jcb.200307137 (2004).Article PubMed PubMed Central Google Scholar 15.Bamias, G., Corridoni, D., Pizarro, T. T. & Cominelli, F. New insights into the dichotomous role of innate cytokines in gut homeostasis and inflammation. Cytokine https://doi.org/10.1016/j.cyto.2012.06.014 (2012).Article PubMed PubMed Central Google Scholar 16.Schultz, G., Clark, W. & Rotatori, D. S. EGF and TGF-α in wound healing and repair. J. Cell Biochem. https://doi.org/10.1002/jcb.240450407 (1991).Article PubMed Google Scholar 17.Laukoetter, M. G. et al. JAM-A regulates permeability and inflammation in the intestine in vivo. J. Cell Biol. https://doi.org/10.1083/jcb1795oia14 (2007).Article Google Scholar 18.Chalaris, A. et al. Critical role of the disintegrin metalloprotease ADAM17 for intestinal inflammation and regeneration in mice. J. Cell Biol. https://doi.org/10.1083/jcb1901oia2 (2010).Article Google Scholar 19.Feng, Y. et al. Loss of ADAM17-mediated tumor necrosis factor alpha signaling in intestinal cells attenuates mucosal atrophy in a mouse model of parenteral nutrition. Mol. Cell Biol. https://doi.org/10.1128/mcb.00143-15 (2015).Article PubMed PubMed Central Google Scholar 20.Uhlen, M., Uhlén, M., Fagerberg, L., et al. Proteomics. Tissue-based map of the human proteome. Science (80- ). Published online 2015.21.The Human Protein Atlas. Published online 2021. https://www.proteinatlas.org/ENSG00000151694-ADAM1722.Yoda, M. et al. Systemic overexpression of TNFα-converting enzyme does not lead to enhanced shedding activity in vivo. PLoS ONE https://doi.org/10.1371/journal.pone.0054412 (2013).Article PubMed PubMed Central Google Scholar 23.Schlöndorff, J., Becherer, J. D. & Blobel, C. P. Intracellular maturation and localization of the tumour necrosis factor α convertase (TACE). Biochem J. https://doi.org/10.1042/0264-6021:3470131 (2000).Article PubMed PubMed Central Google Scholar 24.Endres, K. et al. Tumor necrosis factor-α converting enzyme is processed by proprotein-convertases to its mature form which is degraded upon phorbol ester stimulation. Eur. J. Biochem. https://doi.org/10.1046/j.1432-1033.2003.03606.x (2003).Article PubMed Google Scholar 25.Adrain, C., Zettl, M., Christova, Y., Taylor, N. & Freeman, M. Tumor necrosis factor signaling requires iRhom2 to promote trafficking and activation of TACE. Science https://doi.org/10.1126/science.1214400 (2012).Article PubMed PubMed Central Google Scholar 26.Christova, Y., Adrain, C., Bambrough, P., Ibrahim, A. & Freeman, M. Mammalian iRhoms have distinct physiological functions including an essential role in TACE regulation. EMBO Rep. https://doi.org/10.1038/embor.2013.128 (2013).Article PubMed PubMed Central Google Scholar 27.Calligaris, M. et al. Strategies to target ADAM17 in disease: From its discovery to the iRhom revolution. Molecules https://doi.org/10.3390/molecules26040944 (2021).Article PubMed PubMed Central Google Scholar 28.Li, X. et al. iRhoms 1 and 2 are essential upstream regulators of ADAM17-dependent EGFR signaling. Proc Natl Acad Sci U S A. https://doi.org/10.1073/pnas.1505649112 (2015).Article PubMed PubMed Central Google Scholar 29.Grieve, A. G. et al. Phosphorylation of iRhom2 at the plasma membrane controls mammalian TACE-dependent inflammatory and growth factor signalling. Elife https://doi.org/10.7554/eLife.23968 (2017).Article PubMed PubMed Central Google Scholar 30.Cavadas, M. et al. Phosphorylation of iRhom2 controls stimulated proteolytic shedding by the metalloprotease ADAM17/TACE. Cell Rep. https://doi.org/10.1016/j.celrep.2017.09.074 (2017).Article PubMed PubMed Central Google Scholar 31.Maretzky, T. et al. iRhom2 controls the substrate selectivity of stimulated ADAM17-dependent ectodomain shedding. Proc Natl Acad Sci U S A. https://doi.org/10.1073/pnas.1302553110 (2013).Article PubMed PubMed Central Google Scholar 32.Le Gall, S. M. et al. ADAM17 is regulated by a rapid and reversible mechanism that controls access to its catalytic site. J. Cell Sci. https://doi.org/10.1242/jcs.069997 (2010).Article PubMed PubMed Central Google Scholar 33.Grötzinger, J., Lorenzen, I. & Düsterhöft, S. Molecular insights into the multilayered regulation of ADAM17: The role of the extracellular region. Biochim. Biophys. Acta Mol. Cell Res. https://doi.org/10.1016/j.bbamcr.2017.05.024 (2017).Article PubMed Google Scholar 34.Sommer, A. et al. Phosphatidylserine exposure is required for ADAM17 sheddase function. Nat. Commun. https://doi.org/10.1038/ncomms11523 (2016).Article PubMed PubMed Central Google Scholar 35.Düsterhöft, S., Babendreyer, A., Giese, A. A., Flasshove, C. & Ludwig, A. Status update on iRhom and ADAM17: It’s still complicated. Biochim Biophys. Acta Mol. Cell Res. https://doi.org/10.1016/j.bbamcr.2019.06.017 (2019).Article PubMed Google Scholar 36.Seifert, A. et al. The IRHOM2/ADAM17 axis attenuates bacterial uptake by phagocytes in a cell autonomous manner. Int. J. Mol. Sci. https://doi.org/10.3390/ijms21175978 (2020).Article PubMed PubMed Central Google Scholar 37.Chaohui, C. et al. iRhom2 promotes atherosclerosis through macrophage inflammation and induction of oxidative stress. Biochem Biophys. Res. Commun. https://doi.org/10.1016/j.bbrc.2018.07.133 (2018).Article PubMed Google Scholar 38.Lee, W. et al. IRhom1 regulates proteasome activity via PAC1/2 under ER stress. Sci. Rep. https://doi.org/10.1038/srep11559 (2015).Article PubMed PubMed Central Google Scholar 39.Zhou, Z. et al. Human rhomboid family-1 suppresses oxygen-independent degradation of hypoxia-inducible factor-1a in breast cancer. Cancer Res. https://doi.org/10.1158/0008-5472.CAN-13-1027 (2014).Article PubMed PubMed Central Google Scholar 40.Yan, Z. et al. Human rhomboid family-1 gene silencing causes apoptosis or autophagy to epithelial cancer cells and inhibits xenograft tumor growth. Mol. Cancer Ther. https://doi.org/10.1158/1535-7163.MCT-08-0104 (2008).Article PubMed PubMed Central Google Scholar 41.Gross, A. et al. Desmoglein 2, but not desmocollin 2, protects intestinal epithelia from injury. Mucosal. Immunol. https://doi.org/10.1038/s41385-018-0062-z (2018).Article PubMed Google Scholar 42.Hruz, T. et al. Genevestigator V3: a reference expression database for the meta-analysis of transcriptomes. Adv. Bioinform. https://doi.org/10.1155/2008/420747 (2008).Article Google Scholar 43.Kent, W. J. et al. The human genome browser at UCSC. Genome Res. https://doi.org/10.1101/gr.229102 (2002).Article PubMed PubMed Central Google Scholar 44.Gearing, L. J. et al. CiIIder: A tool for predicting and analysing transcription factor binding sites. PLoS ONE https://doi.org/10.1371/journal.pone.0215495 (2019).Article PubMed PubMed Central Google Scholar 45.Salmon, P. & Trono, D. Production and Titration of Lentiviral Vectors. Curr. Protoc. Neurosci. https://doi.org/10.1002/0471142301.ns0421s37 (2006).Article PubMed Google Scholar 46.Babendreyer, A. et al. Differential induction of the ADAM17 regulators iRhom1 and 2 in endothelial cells. Front. Cardiovasc. Med. https://doi.org/10.3389/fcvm.2020.610344 (2020).Article PubMed PubMed Central Google Scholar 47.Inoue, A. et al. TGFα shedding assay: An accurate and versatile method for detecting GPCR activation. Nat. Methods. https://doi.org/10.1038/nmeth.2172 (2012).Article PubMed Google Scholar 48.Obermeier, F. et al. Interferon-gamma (IFN-γ)- and tumour necrosis factor (TNF)-induced nitric oxide as toxic effector molecule in chronic dextran sulphate sodium (DSS)-induced colitis in mice. Clin. Exp. Immunol. https://doi.org/10.1046/j.1365-2249.1999.00878.x (1999).Article PubMed PubMed Central Google Scholar 49.McIlwain, D. R. et al. iRhom2 regulation of TACE controls TNF-mediated protection against Listeria and responses to LPS. Science https://doi.org/10.1126/science.1214448 (2012).Article PubMed PubMed Central Google Scholar 50.Siggs, O. M. et al. iRhom2 is required for the secretion of mouse TNFα. Blood https://doi.org/10.1182/blood-2012-03-417949 (2012).Article PubMed PubMed Central Google Scholar 51.Issuree, P. D. A. et al. iRHOM2 is a critical pathogenic mediator of inflammatory arthritis. J. Clin. Invest. https://doi.org/10.1172/JCI66168 (2013).Article PubMed PubMed Central Google Scholar 52.Liu, T., Zhang, L., Joo, D. & Sun, S. C. NF-κB signaling in inflammation. Signal Transduct. Target Ther. https://doi.org/10.1038/sigtrans.2017.23 (2017).Article PubMed PubMed Central Google Scholar 53.Lin, Y., Jamison, S. & Lin, W. Interferon-γ activates nuclear factor-κ B in oligodendrocytes through a process mediated by the unfolded protein response. PLoS ONE https://doi.org/10.1371/journal.pone.0036408 (2012).Article PubMed PubMed Central Google Scholar 54.Thapa, R. J. et al. NF-B protects cells from gamma interferon-induced RIP1-dependent necroptosis. Mol Cell Biol. https://doi.org/10.1128/mcb.05445-11 (2011).Article PubMed PubMed Central Google Scholar 55.Shimoda, M. et al. Epithelial cell-derived a disintegrin and metalloproteinase-17 confers resistance to colonic inflammation through EGFR activation. EBioMedicine https://doi.org/10.1016/j.ebiom.2016.02.007 (2016).Article PubMed PubMed Central Google Scholar 56.Dulloo, I., Muliyil, S. & Freeman, M. The molecular, cellular and pathophysiological roles of irhom pseudoproteases. Open Biol. https://doi.org/10.1098/rsob.190003 (2019).Article PubMed PubMed Central Google Scholar 57.Hu, S. et al. TNF-α and IFN-γ synergistically inhibit the repairing ability of mesenchymal stem cells on mice colitis and colon cancer. Am. J. Transl. Res. 11, 6207 (2019).CAS PubMed PubMed Central Google Scholar 58.O’Connell, D. et al. IFN-γ-induced JAK/STAT, but not NF-κb, signaling pathway is insensitive to glucocorticoid in airway epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. https://doi.org/10.1152/ajplung.00099.2015 (2015).Article PubMed PubMed Central Google Scholar 59.Gough, D. J., Levy, D. E., Johnstone, R. W. & Clarke, C. J. IFNγ signaling-Does it mean JAK-STAT?. Cytokine Growth Factor Rev. https://doi.org/10.1016/j.cytogfr.2008.08.004 (2008).Article PubMed Google Scholar 60.Lowenthal, J. W., Ballard, D. W., Bohnlein, E. & Greene, W. C. Tumor necrosis factor α induces proteins that bind specifically to κB-like enhancer elements and regulate interleukin 2 receptor α-chain gene expression in primary human T lymphocytes. Proc. Natl. Acad. Sci. USA. https://doi.org/10.1073/pnas.86.7.2331 (1989).Article PubMed PubMed Central Google Scholar 61.Osborn, L., Kunkel, S. & Nabel, G. J. Tumor necrosis factor α and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor κB. Proc. Natl. Acad. Sci. USA. https://doi.org/10.1073/pnas.86.7.2336 (1989).Article PubMed PubMed Central Google Scholar 62.Matsumiya, T. et al. Characterization of synergistic induction of CX3CL1/Fractalkine by TNF-α and IFN-γ in vascular endothelial cells: an essential role for TNF-α in post-transcriptional regulation of CX3CL1. J. Immunol. https://doi.org/10.4049/jimmunol.0903212 (2010).Article PubMed Google Scholar 63.Geesala, R. et al. Loss of RHBDF2 results in an early-onset spontaneous murine colitis. J. Leukoc Biol. 105(4), 767–781. https://doi.org/10.1002/JLB.4A0718-283RR (2019).CAS Article PubMed Google Scholar 64.Luo, W. W. et al. iRhom2 is essential for innate immunity to RNA virus by antagonizing ER- and mitochondria-associated degradation of VISA. PLoS Pathog. https://doi.org/10.1371/journal.ppat.1006693 (2017).Article PubMed PubMed Central Google Scholar 65.Koff, J. L., Shao, M. X. G., Kim, S., Ueki, I. F. & Nadel, J. A. Pseudomonas lipopolysaccharide accelerates wound repair via activation of a novel epithelial cell signaling cascade. J. Immunol. https://doi.org/10.4049/jimmunol.177.12.8693 (2006).Article PubMed Google Scholar 66.Langer, V. et al. IFN-γ drives inflammatory bowel disease pathogenesis through VE-cadherin–directed vascular barrier disruption. J. Clin. Invest. https://doi.org/10.1172/JCI124884 (2019).Article PubMed PubMed Central Google Scholar Page 2Long-term treatment with DSS leads to induced mRNA expression of iRhom2 and ADAM17 in murine colonic tissue. (A–D) Colon tissue was used from different murine models of colon inflammation. Mice had been either infected with C. rodentium or treated with DSS in drinking water for a short-term or for long-term, respectively. Colon tissues were analyzed for relative mRNA expression of iRhom2 (A), iRhom1 (B), ADAM17 (C) and ADAM10 (D) with mL7 as reference gene by qPCR. (E–F): Analysis of public transcriptome data from human colon tissue samples revealed that iRhom2 is upregulated in mucosal tissue (E) and colon epithelial cells (F) from patients with Crohn’s disease (CD) or ulcerative colitis (UC). Data are shown as mean + SD of at least three independent experiments. Statistical differences in comparison to the control (Ctrl) are indicated by asterisks (* = p ≤ 0.05; ** = p ≤ 0.01; *** = p ≤ 0.001, **** = p ≤ 0.001).
https://www.nature.com/articles/s41598-021-03522-2
Inflammatory activation of surface molecule shedding by upregulation of the pseudoprotease iRhom2 in colon epithelial cells