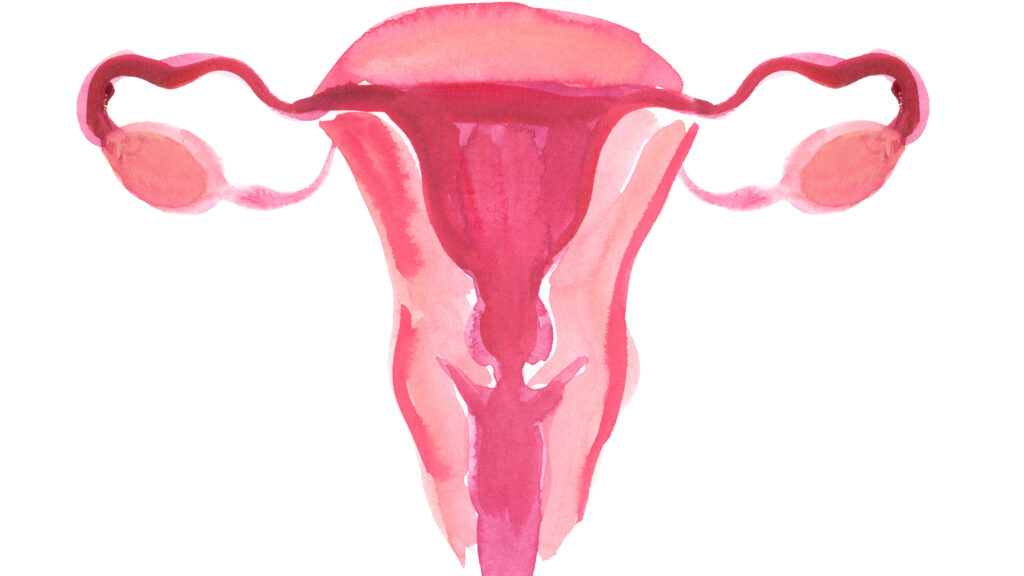
An antibody treatment that delivers a targeted dose of chemotherapy directly to cancer cells shrank tumors in advanced ovarian cancer patients, achieving the primary goal in a late-stage clinical trial, the treatment’s maker, Immunogen, said Tuesday.
Based on the study outcome, Immunogen plans to submit the treatment — a so-called antibody drug conjugate — for accelerated approval with the Food and Drug Administration in early 2022. If cleared, it would be the 40-year-old company’s first wholly owned cancer medicine to reach the market. Unlock this article by subscribing to STAT+ and enjoy your first 30 days free! GET STARTED STAT+ is STAT’s premium subscription service for in-depth biotech, pharma, policy, and life science coverage and analysis. Our award-winning team covers news on Wall Street, policy developments in Washington, early science breakthroughs and clinical trial results, and health care disruption in Silicon Valley and beyond. What’s included? Daily reporting and analysis The most comprehensive industry coverage from a powerhouse team of reporters Subscriber-only newsletters Daily newsletters to brief you on the most important industry news of the day STAT+ Conversations Weekly opportunities to engage with our reporters and leading industry experts in live video conversations Exclusive industry events Premium access to subscriber-only networking events around the country The best reporters in the industry The most trusted and well-connected newsroom in the health care industry And much more Exclusive interviews with industry leaders, profiles, and premium tools, like our CRISPR Trackr. This name will appear with your comment
https://www.statnews.com/2021/11/30/immunogen-study-achieves-primary-goal-of-shrinking-tumors-in-patients-with-ovarian-cancer/