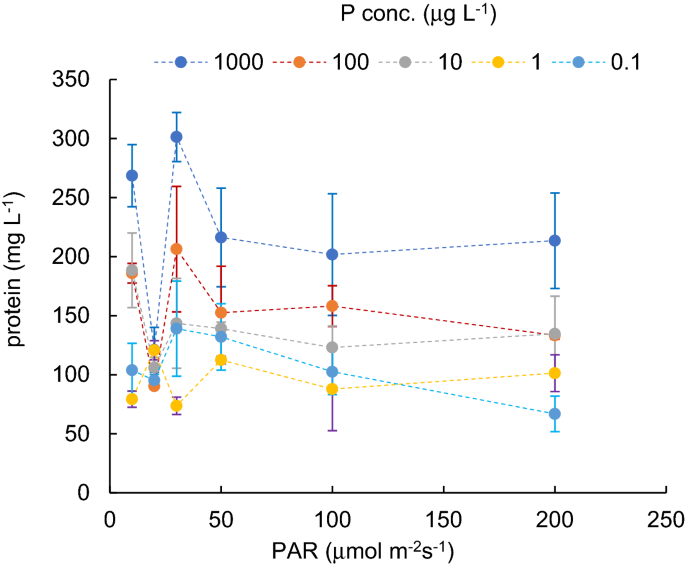
1.Barrington, D. J. & Ghadouani, A. Application of hydrogen peroxide for the removal of toxic cyanobcteria and other phytoplankton from waste water. Environ. Sci. Technol. 4(23), 8916–8921 (2008).ADS Google Scholar 2.Lurling, M., Meng, D. & Fassen, E. L. Effects of hydrogen peroxide and ultrasound on biomass reduction and toxin release in cyanobacterium, Microcytis aeruginosa. Toxins 6(12), 3260–3281 (2014).PubMed PubMed Central Google Scholar 3.Ghime, D. & Ghosh, P. Advanced oxidation processes: A powerful treatment option for the removal of recalcitrant organic compounds. In Advanced Oxidation Processes-Applications, Trends, and Prospects (IntechOpen, 2020).4.Rahdar, S., Igwegbe, C. A., Ghasem, M. & Ahmadi, S. Degradation of aniline by the combined process of ultrasound and hydrogen peroxide (US/H2O2). MethodsX 6, 492–499 (2019).PubMed PubMed Central Google Scholar 5.Derakhshan, Z. et al. Evaluation of kenaf fibers as moving bed biofilm carriers in algal membrane photobioreactor. Ecotoxicol. Environ. Saf. 152, 1–7 (2018).PubMed CAS Google Scholar 6.Shekoohiyan, S. et al. Performance evaluation of cyanobacteria removal from water reservoirs by biological method. Afr. J. Microbiol. Res. 7(17), 1729–1734 (2013).CAS Google Scholar 7.Cooper, W. J., Zika, R., Petasne, R. G. & Plane, J. M. Photochemical formation of hydrogen peroxide in natural waters exposed to sunlight. Environ. Sci. Technol. 22, 1156–1160. https://doi.org/10.1021/es00175a004 (1988).ADS Article PubMed CAS Google Scholar 8.Cooper, W. J., Lean, D. R. S. & Carey, J. H. Spatial and temporal patterns of hydrogen peroxide in lake waters. Can. J. Fish. Aquat. Sci. 46, 1227–1231. https://doi.org/10.1139/f89-158 (1989).Article CAS Google Scholar 9.Cory, R. M. et al. Seasonal dynamics in dissolved organic matter, hydrogen peroxide, and cyanobacterial blooms in Lake Erie. Front. Mar. Sci. https://doi.org/10.3389/fmars.2016.00054 (2016).Article Google Scholar 10.Caverzan, A. et al. Plant responses to stresses: Role of ascorbate peroxidase in the antioxidant protection. Genet. Mol. Biol. 35(4), 1011–1019 (2012).PubMed PubMed Central CAS Google Scholar 11.Sharma, P., Jha, A. B., Dubey, R. S. & Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 1–26 (2012). Google Scholar 12.Ugya, A. Y., Imam, T. S., Li, A., Ma, J. & Hua, X. Antioxidant response mechanism of freshwater microalgae species to reactive oxygen species production: A mini review. J. Chem. Ecol. 36(2), 174–193 (2020).CAS Google Scholar 13.Rastogi, R. P., Singh, S. P., Häder, D.-P. & Sinha, R. P. Detection of reactive oxygen species (ROS) by the oxidant-sensing probe 2′,7′-dichlorodihydrofluorescein diacetate in the cyanobacterium Anabaena variabilis PCC 7937. Biochem. Biophys. Res. Commun. 397(3), 603–607 (2010).PubMed CAS Google Scholar 14.Foyer, C. H. Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ. Exp. Bot. 154, 134–142 (2018).PubMed PubMed Central CAS Google Scholar 15.Gill, S. S. & Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 48(12), 909–930 (2010).PubMed CAS Google Scholar 16.Ma, Z. & Gao, K. Spiral breakage and photoinhibition of Arthrospira platensis (Cyanophyta) caused by accumulation of reactive oxygen species under solar radiation. Environ. Exp. Bot. 68(2), 208–213 (2010).CAS Google Scholar 17.Welkie, D. G. et al. A hard day’s night: Cyanobacteria in diel cycles. Trends Microbiol. 27(3), 231–242 (2019).PubMed CAS Google Scholar 18.Latifi, A., Ruiz, M. & Zhang, C. C. Oxidative stress in cyanobacteria. FEMS Microbiol. Rev. 33(2), 258–278 (2009).PubMed CAS Google Scholar 19.Lea-Smith, D. J., Bombelli, P., Vasudevan, R. & Howe, C. J. Photosynthetic, respiratory and extracellular electron transport pathways in cyanobacteria. Biochim. Biophys. Acta (BBA) Bioenerg. 1857(3), 247–255 (2016).CAS Google Scholar 20.Raja, V., Majeed, U., Kang, H., Andrabi, K. I. & John, R. Abiotic stress: Interplay between ROS, hormones and MAPKs. Environ. Exp. Bot. 137, 142–157 (2017).CAS Google Scholar 21.Asada, S., Fukuda, K., Oh, M., Hamanishi, C. & Tanaka, S. Effect of hydrogen peroxide on the metabolism of articular chondrocytes. Inflamm. Res. 48(7), 399–403 (1999).PubMed CAS Google Scholar 22.Nishiyama, Y. & Murata, N. Revised scheme for the mechanisms of photoinhibition and its application to enhance the abiotic stress tolerance of the photosynthetic machinery. Appl. Microbiol. Biotechnol. 98(21), 8777–8796 (2014).PubMed CAS Google Scholar 23.Mikula, P., Zezulka, S., Jancula, D. & Marsalek, B. Metabolic activity and membrane integrity changes in Microcystis aeruginosa—New findings on hydrogen peroxide toxicity in cyanobacteria. Eur. J. Phycol. 47(3), 195–206 (2012).CAS Google Scholar 24.Huisman, J. & Hulot, F. D. Population dynamics of harmful cyanobacteria. In Harmful Cyanobacteria, 143–176 (Springer, 2005).25.Bergström, A. K. The use of TN:TP and DIN:TP ratios as indicators for phytoplankton nutrient limitation in oligotrophic lakes affected by N deposition. Aquat. Sci. 72(3), 277–281 (2010). Google Scholar 26.Downing, J. A. & McCauley, E. The nitrogen: Phosphorus relationship in lakes. Limnol. Oceanogr. 37(5), 936–945 (1992).ADS CAS Google Scholar 27.Horne, A. J. & Goldman, C. R. Limnology Vol. 2 (McGraw-Hill, 1994). Google Scholar 28.Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 11(1), 15–19. https://doi.org/10.1016/j.tplants.2005.11.002 (2006).Article PubMed CAS Google Scholar 29.Saints, M., Diaz, P., Monza, J. & Borsani, O. Heat stress results in loss of chloroplast Cu/Zn superoxide dismutase and increased damage to Photosystem II in combined drought-heat stressed Lotus japonicus. Physiol. Plant 140(1), 46–56. https://doi.org/10.1111/j.1399-3054.2010.01383.x (2010).Article CAS Google Scholar 30.Suzuki, N., Rivero, R. M., Shulaev, V., Blumwald, E. & Mittler, R. Abiotic and biotic stress combinations. New Phytol. 203(1), 3–43. https://doi.org/10.1111/nph.12797 (2014).Article Google Scholar 31.Asaeda, T. & Barnuevo, A. Oxidative stress as an indicator of niche-width preference of mangrove Rhizophora stylosa. For. Ecol. Manag. 432, 73–82 (2019). Google Scholar 32.Asaeda, T., Senavirathna, M. D. H. J., Vamsi Krishna, L. & Yoshida, N. Impact of regulated water levels on willows (Salix subfragilis) at a flood-control dam, and the use of hydrogen peroxide as an indicator of environmenal stress. Ecol. Eng. 127, 96–102 (2019). Google Scholar 33.Asaeda, T., Senavirathna, M. D. H. J. & Vamsi Krishna, L. Evaluation of habitat preferance of invasive macrophyte Egeria densa in different channel slopes using hydrogen peroxide as an indicator. Front. Plant Sci. 11, 422. https://doi.org/10.3389/fpls.2020.00422 (2020).Article PubMed PubMed Central Google Scholar 34.Diaz, J. & Plummer, S. Production of extracellular reactive oxygen species by phytoplankton: Past and future directions. J. Plankton Res. 40(6), 655–666 (2018).PubMed PubMed Central CAS Google Scholar 35.Drábková, M., Admiraal, W. & Maršálek, B. Combined exposure to hydrogen peroxide and PAR selective effects on cyanobacteria, green algae, and diatoms. Environ. Sci. Technol. 41(1), 309–314 (2007).ADS PubMed Google Scholar 36.Bouchard, J. N. & Purdie, D. A. Effect of elevated temperature, darkness and hydrogen peroxide treatment on oxidative stress and cell death in the bloom-forming toxic cyanobacterium Microcystis aeruginosa. J. Phycol. 47(6), 1316–1325 (2011).PubMed CAS Google Scholar 37.Leunert, F., Eckert, W., Paul, A., Gerhardt, V. & Grossart, H. P. Phytoplanktonic response to UV-generated hydrogen peroxide from natural organic matter. J. Plankton Res. 36(1), 185–197. https://doi.org/10.1093/plankt/fbt096 (2014).Article CAS Google Scholar 38.Wang, B. et al. Optimization method for Microcystis bloom mitigation by hydrogen peroxide and its stimulative effects on growth of chlorophytes. Chemosphere 228, 503–512 (2019).ADS PubMed CAS Google Scholar 39.Foo, S. C., Chapman, I. J., Hartnell, D. M., Turner, A. D. & Franklin, D. J. Effects of H2O2 on growth, metabolic activity and membrane integrity in three strains of Microcystis aeruginosa. Environ. Sci. Pollut. Res. 27(31), 38916–38927 (2020).CAS Google Scholar 40.Barrington, D. J., Reichwaldt, E. S. & Ghadouani, A. The use of hydrogen peroxide to remove cyanobacteria and microcystins from waste stabilization ponds and hypereutrophic systems. Ecol. Eng. 50, 86–94 (2013). Google Scholar 41.Drábková, M., Matthijs, H., Admiraal, W. & Maršálek, B. Selective effects of H2O2 on cyanobacterial photosynthesis. Photosynthetica 45(3), 363–369 (2007). Google Scholar 42.Marsac, N. T. D. Occurrence and nature of chromatic adaptation in cyanobacteria. J. Bacteriol. 130(1), 82–91 (1977). Google Scholar 43.Garcia, P. E., Queimalinos, C. & Dieguez, M. C. Natural levels and photo-production rates of hydrogen peroxide (H2O2) in Andean Patagonian aquatic sysyems: Influence of the dissolved organic matter pool. Chemosphere 217, 550–557 (2019).ADS PubMed CAS Google Scholar 44.Herrmann, R. The daily changing pattern of hydrogen peroxide in New Zealand surface waters. Environ. Toxicol. Chem. 15(5), 652–662 (1996).CAS Google Scholar 45.Spoof, L. et al. Elimination of cyanobacteria and microcystins in irrigation water—Effects of hydrogen peroxide treatment. Environ. Sci. Pollut. Res. 27(8), 8638–8652. https://doi.org/10.1007/s11356-019-07476-x (2020).Article CAS Google Scholar 46.Lopez, C. V. G. et al. Protein measuremements of microalgae and cyanobacterial biomass. Bioresour. Technol. 101(19), 7587–7591 (2010).PubMed Google Scholar 47.Vesterkvist, P. S. M., Misiorek, J. O., Spoof, L. E. M., Toivola, D. M. & Meriluoto, J. A. O. Comparative cellular toxicity of hydrophilic and hydrophobic microcystins on Caco-2 cells. Toxins 4(11), 1008–1023 (2012).PubMed PubMed Central CAS Google Scholar 48.Preece, E. P., Hardy, F. J., Moore, B. C. & Bryan, M. A review of microcystin detections in estuarine and marine waters: Environmental implications and human health risk. Harmful Algae 61, 31–45 (2017).CAS Google Scholar 49.Pham, T.-L. & Utsumi, M. An overview of the accumulation of microcystins in aquatic ecosystems. J. Environ. Manag. 213, 520–529 (2018).CAS Google Scholar 50.Goldman, J. C., McCarthy, J. J. & Peavey, D. G. Growth rate influence on the chemical composition of phytoplankton in oceanic waters. Nature 279(5710), 210–215 (1979).ADS CAS Google Scholar 51.Paerl, H. W., Fulton, R. S. 3rd., Moisander, P. H. & Dyble, J. Harmful freshwater algal blooms, with an emphasis on cyanobacteria. Sci. World. J. 1, 76–113 (2001).CAS Google Scholar 52.Xie, L., Xie, P., Li, S., Tang, H. & Liu, H. The low TN:TP ratio, a case or result of Microcystis blooms?. Water Res. 37(9), 2073–2080 (2003).PubMed CAS Google Scholar 53.Asaeda, T., Rashid, M. H. & Schoelynck, J. Tissue hydrogen peroxide concentration can explain the invasiveness of aquatic macrophytes: A modeling perspective. Front. Environ. Sci. 8, 292 (2021).ADS Google Scholar 54.Hesse, K., Dittman, E. & Borner, T. Consequences of impaired microcystin production for light-dependent growth and pigmentation of Microcystis aeruginosa PCC 7806. FEMS Microbiol. Ecol. 37(1), 39–43 (2001).CAS Google Scholar 55.Tilzer, M. M. Light‐dependence of photosynthesis and growth in cyanobacteria: Implications for their dominance in eutrophic lakes. N. Z. J. Mar. Freshwater Res. 21(3), 401-412 (1987).Article CAS Google Scholar 56.Iwase, S. & Abe, Y. Identification and change in concentration of musty-odor compounds during growth in blue–green algae. J. Mar. Sci. Technol. 8(1), 27–33 (2010). Google Scholar 57.Abeynayaka, H. D. L., Asaeda, T. & Kaneko, Y. Buoyancy limitation of filamentous cyanobacteria under prolonged pressure due to the gas vesicle collapse. Environ. Manag. 60(2), 293–303 (2017).ADS Google Scholar 58.Rippka, R., Deruelles, J., Waterbury, J. B., Herdman, M. & Stanier, R. Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology 111(1), 1–61 (1979). Google Scholar 59.Jana, S. & Choudhuri, M. A. Glycolate metabolism of three submersed aquatic angiosperms during ageing. Aquat. Bot. 12, 345–354 (1982).CAS Google Scholar 60.Veljovic-Jovanovic, S., Noctor, G. & Foer, C. H. Are leaf hydrogen peroxide concentrations commonly overestimated? The potential influence of artefactual interference by tissue phenolics and ascorbate. Plant Physiol. Biochem. 40, 501–507 (2002).CAS Google Scholar 61.Cheeseman, J. M. Hydrogen peroxide concentrations in leaves under natular conditions. J. Exp. Bot. 57(10), 2435–2444 (2006).PubMed CAS Google Scholar 62.Queval, G., Hager, J., Gakiere, B. & Noctor, G. Why are literature data for H2O2 contents so variable? A discussion of potential difficulties in the quantitative assay of leaf extracts. J. Exp. Bot. 59(2), 135–146. https://doi.org/10.1093/jxb/erm193 (2008).Article PubMed CAS Google Scholar 63.Aebi, H. Catalase in vitro. Methods Enzymol. 105, 121–126 (1984).PubMed CAS Google Scholar 64.Nakano, Y. & Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 22(5), 867–880 (1981).CAS Google Scholar 65.Ahmad, P., Jaleel, C. A., Salem, M. A., Nabi, G. & Sharma, S. Roles of enzymatic and non enzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 30(3), 161–175 (2010).PubMed CAS Google Scholar Page 2Protein content for different PAR intensity levels and for each phosphorus concentration level. Vertical bars indicate standard deviation.
https://www.nature.com/articles/s41598-021-02978-6
Hydrogen peroxide can be a plausible biomarker in cyanobacterial bloom treatment