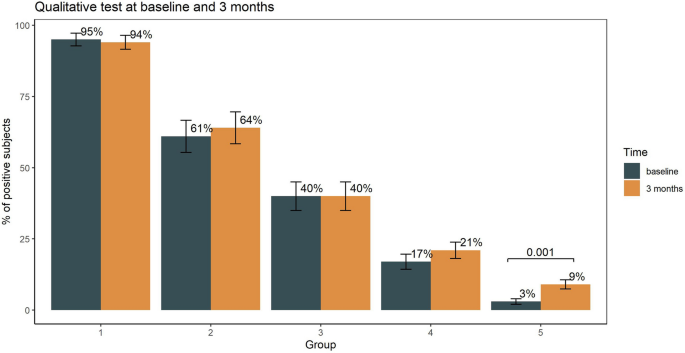
Humoral immune response to SARS-CoV-2 in five different groups of individuals at different environmental and professional risk of infection Silvia Novello1 na1, Massimo Terzolo2 na1, Berchialla Paola orcid.org/0000-0001-5835-56382, Martina Gianetta1, Valentina Bianco1, Francesca Arizio1, Dalila Brero2, Anna Maria Elena Perini2, Adriana Boccuzzi3, Valeria Caramello3, Alberto Perboni4, Fabio Bellavia4 & Giorgio Vittorio Scagliotti1 na1 Scientific Reports 11, Article number: 24503 (2021) Cite this article It is partially unknown whether the immune response to severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection persists with time. To address this issue, we detected the presence of SARS-CoV-2 antibodies in different groups of individuals previously diagnosed with COVID-19 disease (group 1 and 2), or potentially exposed to SARS-CoV-2 infection (group 3 and 4), and in a representative group of individuals with limited environmental exposure to the virus due to lockdown restrictions (group 5). The primary outcome was specific anti-SARS-CoV-2 antibodies in the different groups assessed by qualitative and quantitative analysis at baseline, 3 and 6 months follow-up. The seroconversion rate at baseline test was 95% in group 1, 61% in group 2, 40% in group 3, 17% in group 4 and 3% in group 5. Multivariate logistic regression analysis revealed male gender, close COVID-19 contact and presence of COVID-19 related symptoms strongly associated with serological positivity. The percentage of positive individuals as assessed by the qualitative and quantitative tests was superimposable. At the quantitative test, the median level of SARS-CoV-2 antibody levels measured in positive cases retested at 6-months increased significantly from baseline. The study indicates that assessing antibody response to SARS-CoV-2 through qualitative and quantitative testing is a reliable disease surveillance tool. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is a coronavirus responsible of an acute respiratory disease known as coronavirus disease 2019 (COVID‐19)1,2.At the outbreak of the pandemic, the identification of antigenic structures involved in the immune response and the development of serological diagnostic tests were considered research priorities. Currently, studies of SARS-CoV-2 seroprevalence became progressively relevant to inform public health policies based on the risk of either transmitting or acquiring infection3. It is presently unknown, however, if infection with SARS-CoV-2 in humans protects against future re-infection and, if so, for how long. Common human beta-coronaviruses induce neutralizing antibodies that can last for years and provide protection against re-infection or induce an attenuated disease when individuals get re-infected4. Following COVID 19 disease checking for the presence of anti-SARS-CoV-2 antibodies and assessing the evolution over time of their levels may provide key knowledge to guide individual and population conduct and safety practices5.A recent systematic review included 45 cross-sectional studies that analyzed antibody response mostly in small groups of subjects with different degrees of disese severity during the first 28 days from onset of disease, while only few studies had a follow-up of more than 3 months. Evidence was rated as moderate that most infected individuals develop IgM and IgG anti-SARS-CoV-2 antibodies, with IgG persisting for at least 4 months, while evidence is low on the persistence of neutralizing antibody activity for several months6. Indeed, studies on humoral immunity elicited by SARS-CoV-2 infection generated some debate on its longevity7.The aims of this prospective study were: (1) to assess the presence of SARS-CoV-2 antibodies in different groups of individuals who have been previously diagnosed with COVID-19 disease (group 1 and (2), or potentially exposed to SARS-CoV-2 infection (group 3 and 4), and in a representative group of individuals with limited environmental exposure to the virus (group 5); (3) to assess the the serum persistence over time of SARS-CoV-2 antibodies in individuals with previously confirmed COVID-19 disease.The primary outcome of the study was to assess the presence of specific anti-SARS-CoV-2 antibodies in the different study groups at baseline, at 3 months in all individuals and therefore at 6 months in the individuals who tested positive at the initial or first follow-up serological test. The study was approved by the Institutional Review Board of the San Luigi Hospital (Torino, Italy) in late May 2020.Baseline plasma samples were collected between June 15 and July 31, 2020, when the first wave of epidemic had to a large extent receded. The study was conducted according to the criteria set by the Declaration of Helsinki and each subject signed an informed consent before participating to the study. An online or paper self-administered questionnaire was collected for each participating individual asking for information about SARS-CoV-2 risk exposure, professional role in the hospital, timing of positivity/negativity to nasopharyngeal swab (and, if positive, when a double negative test was obtained), COVID-19 related symptoms and previously diagnosed co-morbidities.Group 1 included individuals with a confirmed diagnosis of SARS-CoV-2 infection by a positive RT-PCR virus test on nasopharyngeal or oropharyngeal swab. Group 2 comprised individuals with suspected SARS-CoV-2 infection due to suggestive clinical features with a negative RT-PCR virus test on at least two nasopharyngeal or oropharyngeal swabs. At the time of the baseline serological test groups 1 and 2 patients were already discharged from hospital and completely recovered. Group 3 included contact or co-exposure with confirmed cases of SARS-CoV-2 infection (household contacts). Group 4 included individuals working in the hospital setting (healthcare workers who were involved in the patient care either in COVID-19 or non-COVID-19 dedicated wards and other occupations) without a positive history for SARS-CoV-2 infection. Group 5 included individuals without occupational risk, living in a geographic area of SARS-CoV-2 outbreak under lockdown restrictions, who did not test positive to the RT-PCR test. For all the enrolled individuals, peripheral blood samples was collected again at month 3 and those tested positive at first and/or second round were tested again at month 6 for longitudinal monitoring of SARS-CoV-2 immune response.As of September 2020, a new assay became commercially available (Elecsys Anti-SARS-CoV-2 S) to measure the quantitative level of antibodies against SARS-CoV-2. All baseline blood samples tested positive at the semiquantitative assay, adequately stored at − 80 °C, were retested with this new assay to compare the qualitative information previously reported with the quantitative assessment.At the 3-month follow up, all the enrolled individuals underwent a second blood test to compare qualitatively baseline results with those at the 3-month follow up and to assess if additional individuals turned up to be positive. At 6-month follow up only individuals positive at baseline and/or at 3-month follow up were tested again with the quantitative test.Secondary outcome measures include the analysis of factors associated with seroconversion and antibody levels both at baseline and during follow-up evaluations.Laboratory investigationPlasma samples adequately stored at – 80 °C were subsequently analyzed in a central laboratory using the Elecsys® Anti‑SARS‑CoV‑2 test an immunoassay for qualitative in vitro detection of antibodies (pan-Ig: IgM, IgG, IGA) to SARS‑CoV‑2 in human serum and plasma using a cobas e801 analyzer (Roche Diagnostics International Ltd, Rotkreuz, Switzerland).Measurement of Anti-SARS-CoV-2 was performed following the manufacturer’s instructions8.The test detects antibodies in serum or plasma, collected using standard sampling tubes. The results are reported as numeric values in form of a cut-off index (COI; signal sample/cutoff) as well as in form of a qualitative results non-reactive (COI < 1.0; negative) and reactive (COI ≥ 1.0; positive).Elecsys® Anti-SARS-CoV-2 S is an immunoassay for the in vitro quantitative determination of antibodies (including IgG) to the SARS-CoV-2 spike (S) protein receptor binding domain (RBD) in human serum and plasma. The linear range of the test is 0.4–250 U/ml. Clinical sensitivity ranges from 88.6% (0–6 days post-PCR confirmation) to 100% (24–36 days) with an analytic specificity of 100% for potential cross-reactive samples, clinical specificity of 100%. The Elecsys® Anti-SARS-CoV-2 S assay was compared to a VSV-based pseudo-neutralization assay in 15 clinical samples from individual patients to assess correlation to serum neutralization capacity9.Sample handlingAnonymized (two side delinked), frozen, residual samples were thawed to room temperature andhomogenized using a slow rotating system or by inverting slowing five times prior to assaying to avoid the production of foam. Before testing, samples were visually inspected to check that they did not contain clots/precipitates, foam or droplets at the container walls. Assay results were obtained via instrument export files.All the diagnostic testing process has been performed by an external, independent laboratory (Life Brain Laboratory, Ovada, Italy) completely blinded of clinical data. The diagnostic tests were kindly provided free of charge by Roche Diagnostics, Italy.Statistical analysisContinuous variables were reported as median and interquartile range (IQR). Categorical variables are reported as number and percentage. Kruskal–Wallis, Chi-square test and Fisher's exact tests were used to compare continuous and categorical variables, as appropriate. Mann–Whitney test with Hochberg adjustment was performed for pairwise comparison between exposure factors and antitboldy levels. Intraclass correlation coefficient was computes as agreement measure between the in vitro quantitative and qualitative assay of antibodies.Multivariable and penalized logistic regression were carried out to test for association between exposure risk factors and (1) antibody levels dichotomized as positive (cutoff index [COI] ≥ 1.0) or negative (COI < 1) response, and (2) positive or negative swab test at baseline. The model selection strategy was based on clinical discussion and statistical automated procedures. The best fitting model was chosen on the basis of the Akaike information criterion and further discussed. Interaction among variables was checked in a similar way. Finally, a multivariate model was built and evaluated using a graphical examination of residual diagnostics. Discrimination Index D (the higher the better) and the Somer concordance index Dxy (the closer to 1 in absolute value the better) were also evaluated.A multilevel quantile regression was used to analyze the association between risk factors for exposure and quantitative antibody levels measured with the Elecsys® Anti‑SARS‑CoV‑2 S test at baseline and 6-month follow up. Median regression was used. The quantile regression was chosen to avoid data transformation due to the non-normal distribution of the errors and 95% confidence intervals were estimated by bootstrapping (500 samples). Random effects on individual were included to account for the repeated measures. Variance–covariance structure of random effects was modeled assuming uncorrelated and Laplace random effects. Goodness-of-fit was assessed through residuals inspection. Statistical significance was set at 5%. All analyses were performed using R version 4.0.2. Default choices were adopted to set up reference groups in multivariable models.The baseline blood samples were collected from 1016 individuals but only 989 were fully eligible for the study because of the incomplete information by the self-administered questionnaire, representing a 97% response rate (supplementary figure S1). Table 1 summarizes the distribution and main characteristics of the 5 considered groups. Median age for the entire study population was 47 years (IQR: 34–58) and women were 59%.Table 1 Main characteristics of the individuals enrolled in the 5 study groups.COVID-19 related symptoms and comorbidities are reported in supplementary table S1. In Group 1 and 2 only 4% of patients were without any symptom. Comorbidities were absent in 55% of the individuals across the 5 groups ranging from 62% in group 4 to 46% in group 2. Most commonly reported co-morbidities included systemic hypertension, previous chronic respiratory and cardiovascular diseases.At baseline, seroconversion rate was 95% in group 1, 61% in group 2, 40% in group 3, and 17% in group 4. Among individuals living under lockdown restrictions (group 5), 3% were seropositive. At the 3-month follow up, the percentage of individuals positive at the qualitative anti‑SARS‑CoV‑2 test was almost unchanged in all the groups with the exclusion of group 5 where the positivity rate increased significantly from 3 to 9% (p = 0.001) (Fig. 1).Figure 1Percentage of subjects who tested positive to qualitative in vitro detection of antibodies at baseline and 3 months follow-up. Statistical differences were examined by McNemar test.Overall, there were 474 healthcare workers, ranging from 41 (group 2) to 141 (group 5). Interestingly, the seroconversion rate among healthcare workers in COVID-19 wards (25%) did not significantly differ from that of physicians working in non-COVID-19 wards (26%). Moreover, the serological test yielded positive results in 10% of physicians with a clinical diagnosis of COVID-19 disease based on suggestive clinical features but tested negative on RT-PCR virus test performed within one week from the hospital admission.Multivariate logistic regression analysis for the qualititative anti-SARS-CoV-2 test dichotomized as positive or negative and taking into account patients' and disease characteristics revealed that male gender, close COVID-19 contact and the presence of COVID-19 related symptoms strongly associated with the positivity a
https://www.nature.com/articles/s41598-021-04279-4
Humoral immune response to SARS-CoV-2 in five different groups of individuals at different environmental and professional risk of infection