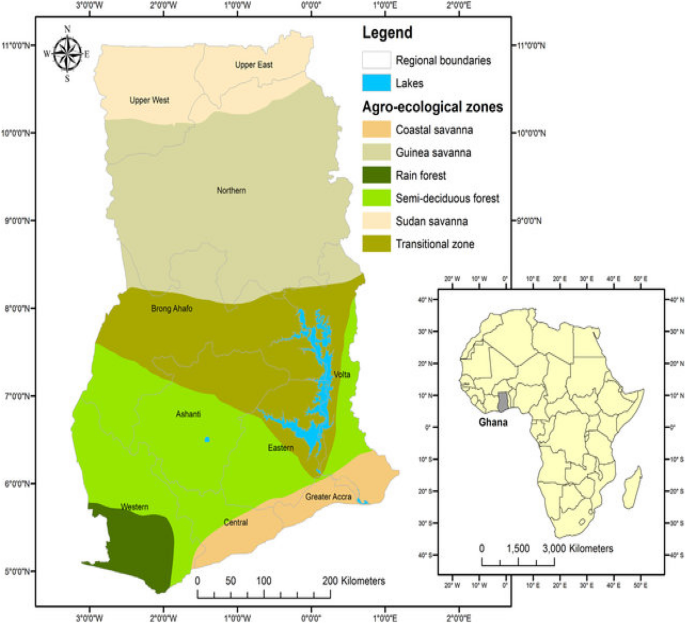
Exposure and risk characterizations of ochratoxins A and aflatoxins through maize (Zea mays) consumed in different agro-ecological zones of Ghana Mycotoxin contamination of foodstuffs is a serious food safety concern globally as the prolonged ingestion of these toxins has the tendency to worsen the risk of hepatocellular carcinoma. This study aimed at estimating ochratoxin A (OTA) and aflatoxin (AF) levels above international (European Food Safety Authority, EFSA) and local (Ghana Standards Authority, GSA) standards as well as the health risks associated with the consumption of maize (n = 180) sampled from six (6) regions representing the agro-ecological zones of Ghana. OTA and AF were measured with High-Performance Liquid Chromatography with a Fluorescence detector. Out of the 180 samples analyzed for total aflatoxins (AFtotal), 131/180 tested positive and 127 (70.50%) exceeded the limits of EFSA and ranged 4.27–441.02 µg/kg. While for GSA, 116 (64.44%) of samples exceeded this limit and ranged between 10.18 and 441.02 µg/kg. For OTA, 103/180 tested positive and 94 (52.22%) of samples between the range 4.00–97.51 µg/kg exceeded the tolerable limit of EFSA, whereas 89 (49.44%) and were in the range of 3.30–97.51 µg/kg exceeded the limits of GSA. Risk assessment values for total aflatoxins (AFtotal) ranged between 50 and 1150 ng/kg bw/day, 0.4–6.67, 0–0.0323 aflatoxins ng/kg bw/day and 1.62–37.15 cases/100,000 person/year for Estimated Daily Intake (EDI), Margin of Exposure (MOE), Average Potency, and Cancer Risks respectively. Likewise, ochratoxin (OTA) values were in the ranges of 8.6 × 10–3–450 ng/kg bw/day, 0.05–2059.97, 0–0.0323 ochratoxins ng/kg bw/day and 2.78 × 10–4–14.54 cases/100,000 person/year. Consumption of maize posed adverse health effects in all age categories of the locations studied since the calculated MOE values were less than 10,000. Maize (Zea mays L), a principal cereal extensively consumed1 around the globe is exceedingly prone to fungal infection by many toxigenic fungal species. This subsequently leads to mycotoxins (fungal toxins) production2,3 due to its ideal nutrient composition4. This situation is particularly distressing since maize (Zea mays) accounts for 40% of the cereal production in Sub-Saharan Africa (SSA), where more than 80% is used as food5. The crop provides at least 30% of the total calorie intake of people in Sub-Saharan Africa. Maize is consumed as a staple in the African region where intake ranges from 52 to 450 g/person/day1,6 and so is an easy channel for mycotoxin contamination. Mycotoxins, which are natural toxins from fungi, contaminate maize grains and render them potentially dangerous. Mycotoxins represent one of the main global foodborne risks for human health, and are considered an important issue in the situation of food safety, due to their acute and chronic toxic effects on animals and humans7,8. Noteworthy, mycotoxins of maize include aflatoxins (AFs), ochratoxin A (OTA), and Fumonisins (FM)9,10,11. Attendant contamination could either be single or occur in multiple combinations with other mycotoxins. Ochratoxins are fungal metabolites produced from the growth of some notable species including Aspergillus ochraceus, A. carbonarius, A. niger, and Penicillium verrucosum. Ochratoxins occur in A, B, C, and D types, of which the most common and toxic type is Ochratoxin A (OTA). OTA is assumed to be one of the five most important mycotoxins in agriculture12. Although not well known in Africa and the world at large compared to aflatoxins, ochratoxins are described as one of the most commonly occurring, mycotoxins in the world due to their presence in a wide variety of foodstuffs such as potatoes, pulses, nuts, spices coffee, cacao, beer, and wine10. Nonetheless, notable among the main foodstuffs are cereals and cereal products, which constitute 60% of its total exposure to man and animals according to13 (JECFA).The toxicological profile of OTA has been investigated and reviewed expansively in numerous studies14,15,16,17,18,19. In summary, these studies showed that OTA is nephrotoxic, hepatotoxic, neurotoxic, teratogenic, mutatoxic, immunotoxic and causes blood–brain barrier damage in various animals and humans, with renal toxicity and carcinogenesis being significant adverse effects. There is limited evidence of OTA-associated chronic kidney disease in humans12. Ochratoxins A (OTA) is aptly classified as a possible human carcinogen (2B agent) according to the International Agency for Research on Cancer (IARC)12,20. Aflatoxins are also fungal metabolites produced by strains of Aspergillus flavus and A. parasiticus. They are by and large the most well-known mycotoxins owing to their persistence in the environment and the ubiquitous nature of their contaminants. Aflatoxins occur in five different types; aflatoxins B1, B2, G1, G2, and M1 produced primarily in cow milk by cow-eating contaminated silage. Prolonged ingestion of aflatoxins has been reported to cause impaired immune function, hepatotoxicity, neurotoxicity, malnutrition, and stunted growth in children, teratogenic, mutagenic disabilities, and eventual death21,22. The International Agency for Research on Cancer (IARC) and the Joint FAO/WHO Expert Committee on Food Additives (JECFA) named it as a class 1 carcinogen due to its high potency23,24.The evidence that mycotoxins can have adverse health effects on humans and animals has led many countries to set up standards for maximum levels of total aflatoxins and ochratoxins in food products intended for both human and animal consumption25. These permissible levels are almost excruciating especially in developing countries in Sub-Saharan Africa. Bankole and Adebanjo26 noted when the worldwide directive of mycotoxins in food is under consideration, the impact seems to be greater on low to medium-income countries. It was projected that enforcement of strict regulations regarding aflatoxin contamination by the European Union would result in the rejection of 64% of imports of cereals, dried fruits, and nuts from African countries with an estimated trade loss per year of approximately US $670 million26. In addition to setting regulatory limits for mycotoxins, it is also imperative to conduct health risk assessments in the population due to dietary exposure. A low-dose extrapolation approach introduced by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) in 1997 and the margin of exposure (MOE) method proposed at the 64th JECFA meeting in 200527 were both recommended and have been widely used worldwide28,29 to assess the risk of dietary exposure to mycotoxins.There is a paucity of data on the combined occurrence of ochratoxin A and aflatoxin contamination in foodstuffs in Ghana. Previous works by national institutions such as the Food and Drugs Authority (FDA), Ghana, Ghana Standards Authority (GSA), and Council for Scientific and Industrial Research (CSIR), Ghana have conducted surveillance programmes to monitor contamination by only aflatoxins in foodstuffs. To the best of our knowledge, not a single work is published on the exposure assessment of the co-occurrence of OTA and AFs as well as their risk characterization. This study is the only study in Ghana that assessed the levels, exposure and risk characterization of ochratoxin A and aflatoxins through the consumption of maize in different age group samples from six (6) different regions representative of the different agro-ecological zones of Ghana by using MOE and quantitative liver cancer risk approaches.To collect a representative data set, we first obtained the list of villages in each district from the Regional Directorate of the Ministry of Agriculture. From each district, an average of 5 villages (Table 1) was then randomly selected. The maize sellers in each market were conveniently sampled where about one kilogram (1 kg) of raw maize samples were purchased concurrently from July to December 2020. Five hundred (500) grams each of maize samples were fetched and kept in sterile bags in ice chests and sent to the laboratory within the same day in a vehicle where they were stored in a deep freezer at − 20 °C until ready for chemical analysis30.Table 1 Geographical locations and some attributes of the origin of samples.Determination of Ochratoxins AChemicals and standardsThe analytical standard of OTA was supplied by Sigma-Aldrich (St. Louis, MO, USA). All solvents used for the preparation of the mobile phase were HPLC grade and obtained from Merck (Darmstadt, Germany). Methanol and hexane used for extraction were of analytical grade supplied by Sigma-Aldrich. All homogenized mixtures and eluates were filtered through Whatman no. 4 and 0.45 mm membrane filters respectively (Whatman plc, Maidstone, UK). De-ionized water was obtained with a Millipore Elix Essential purification system (Bedford, MA, USA). OCHRA PREP immunoaffinity columns were supplied by R-Biopharm, Rhone limited, and used for SPE and cleanup. These columns have a concentration capacity of 100 ng/mL with at least 90% recovery. Phosphate-buffered saline (PBS) was prepared by dissolving PBS tablets (Sigma-Aldrich) in distilled water. Sodium chloride (≥ 99.0%) was sourced from Sigma-Aldrich. Six-point calibration was made using the pure Ochratoxin A standard at concentrations of 5 µg/kg, 10 µg/kg, 15 µg/kg, 20 µg/kg, 25 µg/kg and 30 µg/kg. Linearity was accepted at 0.99 or 99% for the regression curve (CEN official method EN1412333).Determination of OchratoxinOchratoxin A was determined based on CEN official method EN14123 (2007)32. About 500 g each of maize was sampled by thoroughly mixing and heaping the whole batch into a cone. Using cardboard, the heap was divided into four equal parts. Two opposite parts were mixed, and the remaining two parts were packed, and the process repeated until a representative 500 g sample was achieved and ground into fine maize powder and groundnut slurry. Exactly 25 g of powdered or slurred samples were extracted with 5 g Sodium Chloride and 200 mL methanol in distilled water in a ratio of 4:1, respectively. Hexane (100 mL) was added to the groundnut mixture and the samples homogenized for 3 min (i.e., 3000 rpm for 2 min and at 3500 rpm for 1 min). The groundnut mixture generated two organic layers (the hexane upper layer and methanol lower layer). The lower methanol layer of the groundnut mixture and the maize mixture was filtered through Whatman number 4 filter paper. Ten milliliters (10 mL) of filtrate were used for Ochratoxin A solid-phase extraction and cleanup. Exactly 150 mL of phosphate buffer saline (PBS) was added to 10 mL of filtrate and the mixture stirred. Immuno-affinity columns specific for Ochratoxin A were pre-conditioned and antibodies in the column were activated by eluting 10 mL of phosphate buffer saline through columns at a flow speed of 3 mL/min. Exactly 50 mL of the 160 mL filtrate-PBS mixture was loaded onto pre-conditioned immune-affinity columns specific for Ochratoxin A and allowed to drain by gravity. The columns were washed three times with 5 mL PBS and allowed to elute at a flow rate of 5 mL/min. Using a vacuum pump, the air was blown through the columns to get rid of all wash solvent molecules. Ochratoxin A was eluted in two steps into a 5 mL volumetric flask by first eluting with 1 mL of methanol (highest grade) followed by another 1 mL of methanol after one minute. Air was blown through the column to collect all eluates. Aqueous acetic acid (1%) was used to make up the volume of eluate to 4 mL and eluate vortexed, after which 2 mL was pipetted into HPLC vials for quantification.HPLC parametersAgilent high-performance liquid chromatography system (HPLC 1260 infinity series) with a quaternary pump and fluorescence detection was used for OTA quantification. Data acquisition and quantification were done using Chem station (Open Lab edition). The Agilent HPLC equipped with a fluorescence detector was set at an excitation wavelength of 333 nm and an emission wavelength of 467 nm and the column compartment temperature regulated at 30 °C. The mobile phase was a mixture of 5 mM sodium acetate with acetic acid (pH 2.4): methanol: acetonitrile at ratios of 40:30:30, respectively, and an isocratic delivery mode employed at a flow rate of 1 mL/min with an injection volume of 10 µl. The run time was set at 10 min (CEN official method EN1412333).Aflatoxins determinationExtraction of samplesAFB1, AFB2, AFG1, and AFG2 were extracted from the samples according to the European Committee for Standardization (CEN) official method EN1412333 for aflatoxin extraction. Methanol in water (200 mL) (8 + 2) and 5 g NaCl were used to extract 25 g of sample. Hexane (100 mL) was added to samples containing more than 50% fat. The mixture was homogenized for 3 min at 3000 rpm (2 min) and 3500 rpm (1 min). The extracts were filtered and 10 mL of the filtrate added to 60 mL of phosphate buffer saline (PBS) for solid-phase extraction using a preconditioned immune-affinity column specific for, AFB1, AFB2, AFG1, and AFG2. The 70 mL filtrate-PBS mixture was loaded onto the preconditioned column and allowed to elute by gravity at a flow rate of 1 mL/min. This was followed by a cleanup with 15 mL distilled water at a flow rate of 5 mL/min. Aflatoxins were eluted in two steps into a 5 mL volumetric flask with 0.5 mL followed by 0.75 mL of methanol (HPLC grade) and allowed to elute by gravity. Deionized water was used to make up the volume of eluate to 5 mL and eluate vortexed and 2 mL pipetted into HPLC vials for quantification.HPLC parametersInjection volume: 10 μl flow rate: 1 mL/min, column temperature: 35 ℃, excitation wavelength: 360 nm, emission wavelength: 440 nm, mobile phase composition: water/acetonitrile/MeOH (65:15:20 v/v/v), post-column derivatization: Kobra cells. HPLC Column Specification Spherisorb ODS1- Excel (4.6 mm × 25 cm), 5 μm particle size, 250 A pore size LOD = Limit of detection LOQ = Limit of quantification ACN = Acetonitrile MeOH = Methanol LOD calculation = 3 * standard deviation/slope LOQ calculation = 3 × LOD Supplier of Column R- Biopharm, Block 10 campus, West Scotland Science Park, Acre Road, Glasgow, Scotland G20 OXA Analysis of samplesThe aflatoxins (by Aspergillus flavus and A. parasiticus) levels in the samples were determined according to the CEN official method EN1412333 by High-Performance Liquid Chromatography HPLC (Agilent 1260 Series, OpenLab software, X-bridge column) (250 mm × 4.6 mm, i.d., 5 μm), USA with fluorescence detector and post-column derivatization using Kobra cells to generate bromine electrochemically at the CSIR- Food Research Institute, Ghana.LOD for Ochratoxins was 0.83 µg/kg while LOQ recorded 2.49 µg/kg (Table 2).Table 2 Limits of Detection (LOD) and Quantification (LOQ) of ochratoxins (OTA) and aflatoxins (AFB1, AFB2, AFG1, AFG2, and AFtotal) (µg/kg) measured by HPLC.LOD for AFB1, AFB2 was 0.15 µg/kg, and 0.13 µg/kg for AFG1 and AFG2, while LOQ were 0.39 and 0.45 µg/kg (Table 2).Limit of detection/quantification (LOD/LOQ)Limits of detection and quantification (LOD/LOQ) of the HPLC were estimated by making a calibration curve around the standard used for spiking, 5 µ/kg (the lowest concentration range of the calibration curve). Blank did not produce any signal, so the LOD and LOQ were calculated as;$$ {text{LOD}} = {3}*{text{standard}},{text{deviation}}/{text{slope}}. $$$$ {text{LOQ}} = {3}*{text{LOD}}. $$Measurement accuracySpiking of pure aflatoxin standard solution was done to ensure the measurement accuracy of the analysis. Three levels of spiking were done at the lower, mid, and upper concentration range of the calibration curve concentrations (5 ppb, 15 ppb, and 30 ppb). Spike volumes of pure standards were calculated as;$$ left[ {{tex
https://www.nature.com/articles/s41598-021-02822-x
Exposure and risk characterizations of ochratoxins A and aflatoxins through maize (Zea mays) consumed in different agro-ecological zones of Ghana