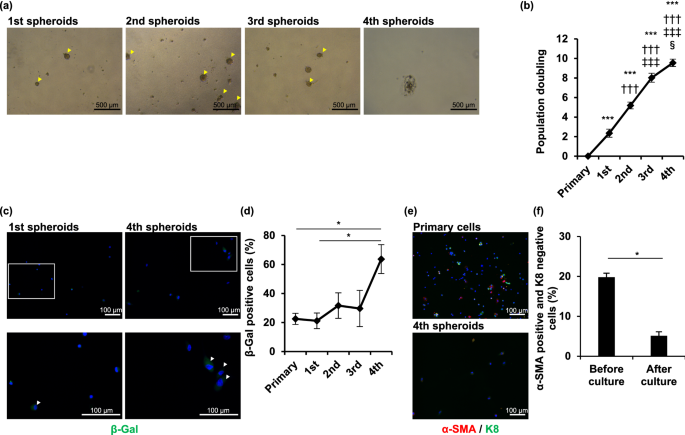
Establishment and characterization of immortalized sweat gland myoepithelial cells Sweat glands play an important role in thermoregulation via sweating, and protect human vitals. The reduction in sweating may increase the incidence of hyperthermia. Myoepithelial cells in sweat glands exhibit stemness characteristics and play a major role in sweat gland homeostasis and sweating processes. Previously, we successfully passaged primary myoepithelial cells in spheroid culture systems; however, they could not be maintained for long under in vitro conditions. No myoepithelial cell line has been established to date. In this study, we transduced two immortalizing genes into primary myoepithelial cells and developed a myoepithelial cell line. When compared with primary sweat gland cells, the immortalized myoepithelial cells (designated “iEM”) continued to form spheroids after the 4th passage and expressed α-smooth muscle actin and other proteins that characterize myoepithelial cells. Furthermore, treatment with small compounds targeting the Wnt signaling pathways induced differentiation of iEM cells into luminal cells. Thus, we successfully developed an immortalized myoepithelial cell line having differentiation potential. As animal models are not useful for studying human sweat glands, our cell line will be helpful for studying the mechanisms underlying the pathophysiology of sweating disorders. The eccrine sweat gland, an exocrine gland in the skin, plays a pivotal role in the thermoregulation of the human body through sweat secretion. The eccrine sweat gland is a single tube composed of a secretory coil and a duct tube. The secretory coil consists of myoepithelial and secretory luminal cells, and plays a major role in the production and delivery of sweat to the skin surface1. Myoepithelial cells have been hypothesized to regulate sweat secretion through the contraction of secretory coils2, whereas secretory luminal cells release sweat into the lumen of secretory coils3. Myoepithelial cells in secretory coils play a pivotal role in maintaining sweat glands through self-renewal and differentiation into luminal cells4,5.Myoepithelial cells highly express α-smooth muscle actin (SMA) that contributes to the generation of contractile force during sweat secretion2. This contraction is thought to be induced by acetylcholine via cholinergic receptor M3, which is highly expressed in myoepithelial cells3. Myoepithelial cells express the integrin α6β1 and keratin 14 (K14) genes, which are also expressed in the stem cells of the interfollicular epidermis and mammary gland. However, myoepithelial cells do not express keratin 8 (K8) and 18 (K18), which are expressed in secretory luminal cells5.To further understand the physiology of sweat glands, long-term in vitro culturing of myoepithelial cells is a promising model. However, myoepithelial cells cannot be maintained in a two-dimensional monolayer culture system; only a spheroid culture system without cell adhesion can maintain myoepithelial cells up to the third passage5,6. Mammary gland cells, another type of well-studied exocrine gland cells, also require a spheroid suspension culture system to preserve their stemness characteristics, although these cells fail to form spheroids after the fourth passage7. This limitation was circumvented using immortalized mammary gland cells by transduction of two immortalizing genes such as human telomerase reverse transcriptase (hTERT) and the Simian virus 40 large T and small t antigen (SV40Tt), which can be maintained in a spheroid culture system beyond the fourth passage8.In the present study, we aimed to establish immortalized myoepithelial cells in a spheroid culture system using the same approach. We found that the spheroid culture system could not maintain myoepithelial cells after the third passage. Therefore, we established immortalized myoepithelial cells overexpressing hTERT and SV40Tt. These immortalized cells retained myoepithelial cell phenotypes, including their ability to differentiate into luminal cells.To assess the long-term spheroid formation ability of human sweat gland cells in vitro, we first performed serial passage culture of sweat gland cells under spheroid suspension conditions. Consistent with a previous report5, sweat gland cells formed spheroids and proliferated until the third passage, but the cell aggregates shrank and became nonspherical thereafter (Fig. 1a,b and Supplementary Fig. S1a). Because cell proliferation arrest is commonly caused by the induction of cellular senescence, a state of irreversible cell cycle arrest in cultured cells, we assessed the accumulation of the senescent cells in the serial passage of sweat gland cells. The number of β-gal-positive sweat gland cells at the fourth passage was significantly higher than that at the first passage and before the spheroid culture (P = 0.018 and 0.022) (Fig. 1c,d and Supplementary Fig. S1b). We have previously reported that myoepithelial cells are stem cells in sweat glands, which proliferate and form spheroids5,6. To determine the proportion of myoepithelial cells in total sweat gland cells, we examined the number of α-SMA-positive cells in the primary sweat gland cells and those arrested in spheroid formation. The proportion of α-SMA-expressing cells significantly decreased when the cells failed to form spheroids (P = 0.023) (Fig. 1e,f). These results indicated that the in vitro spheroid culture of human sweat gland cells cannot be maintained for more than the fourth passage under spheroid suspension conditions. Moreover, the lack of cell proliferation and spheroid formation might be caused by a reduction in the proliferative ability of myoepithelial stem cells after serial passages in spheroid cultures.Figure 1Serially passaged primary sweat gland cells undergo spheroid formation arrest. (a) Each image shows sweat gland cell spheroids from the 1st to 4th generation. The cells were cultured in suspension and passaged every seven days. Arrowheads indicate spheroids. (b) Growth curves of sweat gland cells derived from five different patients in each generation. Data are presented as the mean ± standard error (S.E.) of five independent experiments (n = 5). §P < 0.05; ***, †††, ‡‡‡, P < 0.001 (*,**versus primary, †† versus 1st spheroids, ‡‡ versus 2nd spheroids, § versus 3rd spheroids). (c) Images show β-galactosidase (β-gal) staining of cells at the 1st and 4th generation of spheroids. Arrowheads indicate β-gal-positive cells. The bottom panels show magnified views of the areas shown in the upper panels. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI). (d) Rate of β-gal-positive cells derived from spheroids of each generation. At least 50 cells were counted in each experiment. Data are presented as the mean ± S.E. of five independent experiments (n = 5). *P < 0.05. (e) Immunofluorescence staining of cells derived from sweat gland tissues (primary cells) and 4th generation spheroids (4th spheroids) for α-smooth muscle actin (α-SMA) (red) and keratin 8 (K8) (green). Nuclei were stained with DAPI. (f) Rate of α-SMA-positive and K8-negative cells before and after the culture until spheroid formation arrest. At least 50 cells were counted in each experiment. Data are presented as the mean ± S.E. of five independent experiments (n = 5). * P < 0.05. Scale bars: 500 μm in A and 100 μm in b and c.Introduction of immortalizing genes prolongs the cell culture period in sweat glandsAs the growth of sweat gland cells under in vitro culture was disturbed by spontaneous cellular senescence, primary sweat gland cells could not be cultured for a long time. According to a previous study on glandular cell immortalization8, we attempted to immortalize sweat gland cells by the transduction of the hTERT and SV40Tt genes (Fig. S2). The hTERT- and SV40Tt-transduced cells displayed spheroid formation at the fourth and ninth passages (Fig. 2a), and were competent in forming spheroids even after the 19th passage, on average (Fig. 2b). Population doubling was also significantly increased in the hTERT- and SV40Tt-transduced cells, capable of population doubling 65 times compared with 7 times in untransduced cells (P < 0.001) (Fig. 2c). In contrast, spheroid formation and population doubling in single gene-transduced cells were not significantly different from those in the untransduced cells. The hTERT- and SV40Tt-transduced cells formed a spherical structure until day 4 and the spheroids size continued to increase thereafter (Supplementary Fig. S3). Furthermore, the cryopreserved hTERT-and SV40Tt-transduced cells were capable of forming spheroids and competent in passaging several times (Supplementary Fig. S4a). Moreover, the proportion of β-gal-positive senescent cells in hTERT- and SV40Tt-transduced cells was significantly lower than that in the untransduced control cells at the fourth or fifth passage when these cells failed to form spheroids (P < 0.001) (Fig. 2d,e). This indicated that we successfully generated immortalized sweat gland cells in the suspension spheroid culture system.Figure 2Establishment of immortalized sweat gland cells in spheroid culture. (a) Spheroids formed from sweat gland cells transduced with human telomerase reverse transcriptase (hTERT) and Simian Virus 40 large T and small t antigen (SV40Tt) genes at the indicated generation of spheroids. The cells were passaged once daily for seven days. Arrowheads indicate spheroids. (b,c) Number of passages (b) and population doublings (c) in spheroid culture of sweat gland cells transduced with hTERT and/or SV40Tt genes. Data are presented as the mean ± standard error (S.E.) of five independent experiments (n = 5). ***P < 0.001. (d) β-galactosidase (β-gal) staining of sweat gland cells transduced with hTERT + SV40Tt and untransduced cells at the 4th spheroid. Arrowheads indicate β-gal-positive cells. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI). (e) Rate of β-gal-positive cells in hTERT + SV40Tt transduced cells and untransduced cells. At least 50 cells were counted under each condition. Data are presented as the mean ± S.E. of five independent experiments (n = 5). ***P < 0.001. Scale bars: 500 μm in A and 100 μm in d.Immortalized sweat gland cells maintain myoepithelial cell propertiesAs immortalized sweat gland cells were competent in spheroid formation, which was also observed in the culture of myoepithelial cells5, we examined whether the immortalized cells expressed myoepithelial cell-specific protein. α-SMA-positive cells were predominantly observed in the immortalized cells at the fourth passage, whereas α-SMA-positive cells were hardly observed in the untransduced control cells (P < 0.001) (Fig. 3a,b). The immortalized cells retained α-SMA-positive cells after further passages (e.g., at the sixth passage) and did not produce K8, a luminal cell marker (Fig. 3c). Immortalized cells retained α-SMA production even after cryopreservation (Supplementary Fig. S4b). Moreover, the majority of immortalized sweat gland cells at the sixth passage indicated the presence of α-SMA with integrin β1 or K14, similar to those in the primary myoepithelial cells (Fig. 3d,e). These results strongly indicated that our sweat-gland-cell-derived immortalized cells retained myoepithelial-cell-specific protein production. Therefore, we termed the generated cells 'immortalized eccrine sweat gland myoepithelial (iEM) cells.'Figure 3Immortalized sweat gland cells exhibit myoepithelial cell properties. (a) Immunofluorescence of human telomerase reverse transcriptase and Simian Virus 40 large T and small t antigen (hTERT + SV40Tt) gene-transduced cells and untransduced cells in the 4th spheroids for α-smooth muscle actin (α-SMA) and keratin 8 (K8). (b) Rate of α-SMA-positive and K8-negative cells in hTERT + SV40Tt gene-transduced cells and untransduced cells at the 4th spheroids. At least 50 cells were counted under each condition. Data are presented as the mean ± S.E. of five independent experiments (n = 5). ***P < 0.001. (c–e) Immunofluorescence of hTERT + SV40Tt-transduced cells in the sixth spheroid, blood cells (negative control), and primary sweat gland cells (primary sweat gland cells; positive control) for α-SMA and K8 (c), α-SMA and integrin β1 (d), and α-SMA and keratin 14 (K14) (e). Arrowheads indicate α-SMA-positive cells (a), α-SMA and integrin β1 positive cells (d), and α-SMA and K14 positive cells (e). The second panels from the left are magnified views of the boxed areas in the left panels. Scale bars: 100 μm.iEM cells can differentiate into luminal cellsTo confirm whether iEM cells are capable of differentiation, similar to primary myoepithelial cells, we cultured iEM cells in serum-containing spheroid culture medium used for inducing differentiation of glandular stem cells in the mammary and meibomian glands7,9. Serum-supplemented culture medium reduced α-SMA and K14 production in iEM cells, whereas serum-free culture medium did not (Fig. 4a). Additionally, adherent culture conditions, which induced the loss of myoepithelial cells in primary sweat gland cells, also reduced α-SMA and K14 gene expression in iEM cells (Supplementary Fig. S5a). Thus, iEM cells were responsive to serum as the cell differentiation stimulus, which was identical to mammary gland stem cells8. However, this culture medium-containing serum did not show clear differentiation of iEM cells into luminal cells, and K8 expression was not detected (Supplementary Fig. S5b). Therefore, we attempted to find a suitable stimulation for the differentiation of iEM cells into luminal cells. We screened small compounds capable of differentiating iEM cells into luminal cells from a panel of small compounds (differentiation inhibitors and activators), by employing K18 production as the marker for luminal cell production. Thirty-four compounds were found to increase K18 mRNA expression in iEM cells (Table 1). Among these, ICG-001, a Wnt signaling pathway inhibitor, increased the production of K8, another luminal-cell-specific protein, in iEM cells (Fig. 4b,c). We performed immunofluorescence staining to assess K8 and α-SMA protein production in ICG-001-treated iEM cells. K8-producing cells were found in iEM cells treated with ICG-001 but not in the control cells (Fig. 4d). These results indicated that iEM cells are competent in differentiating into luminal cells when small compounds, including ICG-001, are provided. Therefore, iEM cells possess myoepithelial cell characteristics, including specific protein expression and differentiation potential, similar to primary myoepithelial cells.Figure 4Immortalized eccrine sweat gland myoepithelial cells retain the potential of differentiation into luminal cells. (a) Immunofluorescence of immortalized myoepithelial (iEM) cells cultured with or without 10% fetal bovine serum (FBS) for α-smooth muscle actin (α-SMA) and keratin 14 (K14) for seven days. The second panels from the left are magnified views of the boxed areas in the left panels. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI). (b) Immunoblots of iEM cells treated with 10 μM ICG-001 (right) and dimethylsulfox
https://www.nature.com/articles/s41598-021-03991-5
Establishment and characterization of immortalized sweat gland myoepithelial cells