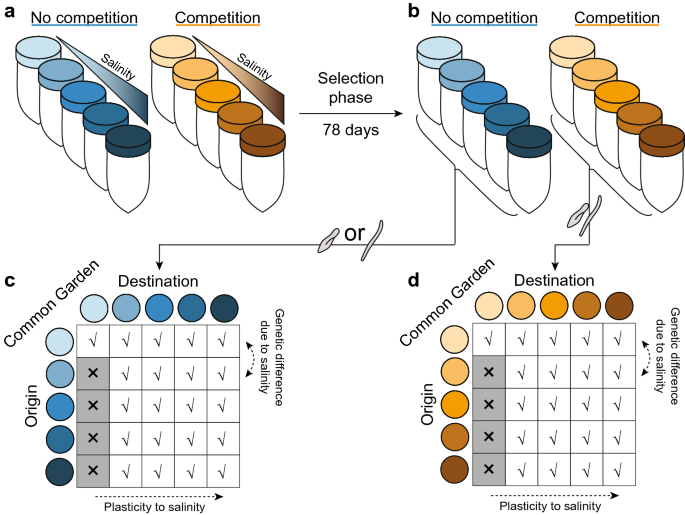
Competition alters species’ plastic and genetic response to environmental change Species react to environmental change via plastic and evolutionary responses. While both of them determine species’ survival, most studies quantify these responses individually. As species occur in communities, competing species may further influence their respective response to environmental change. Yet, how environmental change and competing species combined shape plastic and genetic responses to environmental change remains unclear. Quantifying how competition alters plastic and genetic responses of species to environmental change requires a trait-based, community and evolutionary ecological approach. We exposed unicellular aquatic organisms to long-term selection of increasing salinity—representing a common and relevant environmental change. We assessed plastic and genetic contributions to phenotypic change in biomass, cell shape, and dispersal ability along increasing levels of salinity in the presence and absence of competition. Trait changes in response to salinity were mainly due to mean trait evolution, and differed whether species evolved in the presence or absence of competition. Our results show that species’ evolutionary and plastic responses to environmental change depended both on competition and the magnitude of environmental change, ultimately determining species persistence. Our results suggest that understanding plastic and genetic responses to environmental change within a community will improve predictions of species’ persistence to environmental change. The rate of anthropogenically-induced environmental change is nowadays faster than ever1, including changes in temperature and precipitation2, pollution by nutrients3, salts4, and synthetic chemicals5. Such rapid changes in abiotic factors have resulted in losses of biodiversity and associated services6,7. Previous studies have shown how individual species have the ability to phenotypically track environmental changes or to migrate to novel habitats in order to survive8,9. Phenotypic tracking of environmental conditions occurs either via phenotypic plasticity or via evolution. Phenotypic plasticity is the capacity of a single genotype to produce a range of phenotypes under varying environments10. It is a within-generation individual response mechanism that can occur rapidly to enable short-term survival of organisms to changing environments11,12. Evolution is the genetic adaptation through mutations or via the selection of existing genotypes13. Evolution occurs across generations and therefore is often a slower response to environmental change, nevertheless, crucial for long-term survival of species11,12.Predicting species’ responses to environmental change requires partitioning phenotypic responses into its plastic and genetic components14,15. Yet, species’ plastic and genetic responses to environmental change are mostly studied in isolation of each other12,16, in a single-species perspective17,18, or in simple environmental contrasts (i.e. a control versus a single treatment condition only19). It is expected that the direction and magnitude of plasticity and evolutionary trait change would differ depending on the magnitude of environmental change, and whether selection occurred within a multi-species setting such as a community as opposed to a single species15.In virtually all natural systems, organisms of different species are embedded in communities, in which direct and indirect ecological effects among species can also exert selection pressures on each member of the community20,21, for example through competition for limiting resources22 or space19. Previous studies have shown that species interactions (such as competition, predation, or parasitism) can influence species’ responses to environmental change19,22,23,24 and that this effect can even depend on the abiotic environment21. These studies have shown that species respond differently to a novel environment depending whether they occur alone or in the presence of other species22. This, because the presence of other species most likely changes the fitness landscape in which they evolve19. Species interactions have also been found to induce phenotypic plasticity as well as evolutionary responses in the focal species (e.g. predator-induced defenses25 or competition26). More recently, Grainger et al.27 showed that interspecific competition can even have legacy effects further determining species’ evolutionary responses to environmental change. To our knowledge, no study has explicitly quantified the relative extent to which plasticity and evolutionary changes contribute to the observed phenotypic responses to environmental change when species are embedded within a community. Therefore, it remains largely unknown whether species interactions, such as interspecific competition, can alter the plastic and genetic response to the abiotic change, whether the effect of interspecific competition depends on the magnitude of the abiotic change, and whether this effect varies among different member species of the community.Here, we used three naturally co-occurring and competing freshwater ciliate species28 (Paramecium aurelia, Spirostomum teres, and Tetrahymena thermophila) to experimentally quantify plastic and genetic responses to abiotic environmental change (here: salinity) within the absence and presence of competing species. We use freshwater ciliates as model organisms because they are easy to maintain and manipulate in the laboratory, and they have short generation times. There also exists standardized protocols and automated methods for quantification and phenotyping of these organisms, which allows addressing a wide range of ecological and evolutionary questions29. Moreover, freshwater ciliates and other protists are a major part of the global biomass30, and play a key role in the aquatic food web by providing nutrition for higher trophic levels31, and by being important grazers of bacteria32. The species used are known to be naturally competing for similar resources, and have a life history and ecology that makes them suitable for such comparisons (see also29). The use of salt as a stressor reflects the increasing global problem of salinization of freshwater systems33,34. It is expected that its devastating effects on freshwater systems will intensify with increasing climate change35.We conducted an experiment in two phases. First, we performed a long-term selection experiment exposing replicated populations of each species or replicated communities of all three species to an environmental gradient of increasing salinity (five levels). This resulted into 20 unique experimental treatment combinations (i.e. 5 salinity environments × 4 species treatments; each replicated 3 times), run for 78 days (corresponding to at least 50–150 generations). From the selection phase we can determine whether and how interspecific competition alters phenotypic trajectories to abiotic environmental change varying in magnitude.Then, to disentangle plastic and genetic components of observed trait changes, the populations and communities at the end of the selection phase (further referred to as the selected populations) were used in a common garden experiment (Fig. 1). In this common garden experiment, each selected population or community was exposed to each of the different salt concentrations used in the selection phase (except for the zero salinity selected populations due to the impossibility of transferring individuals without some of the salty media they are embedded in). From the common garden experiment, we can assess (1) whether abiotic environmental change varying in magnitude results in different plastic and genetic responses of the populations evolved without competition and (2) whether these responses are altered by competition. By using the entire community in a common garden (versus singling out the individual species), we can evaluate whether the presence of competing species can mask plastic or genetic responses to abiotic environmental change. When competition between species is weak, plastic and genetic responses to abiotic environmental change in the absence and presence of competition would be comparable17. However, in cases of strong competition, we might expect plastic and genetic responses to environmental change to be accelerated or inhibited when competition selects or does not select for similar trait values in the new environment36.Figure 1Experimental set-up. (a) During the selection phase, populations of all species were kept in five different environmental conditions (represented by color shades) either in absence (blue colors) or presence (orange colors) of competing species (each threefold replicated). (b) End of the selection phase. Change in trait means from (a) to (b) reflect the phenotypic temporal response to salinity and competition. (c,d) Common garden experiment in which aliquots of the populations of all species evolved in the absence (c) and presence (d) of competition are placed in a common salinity environment, respectively. In this study, for the competition treatment an aliquot of the end point of the selection phase was used in the common garden. Comparing trait means between (c) and (d) shows whether the response to salinity depends on the competition and salinity condition the species was selected in.As multiple species were used, we can additionally assess whether patterns found vary among species. Consequently, we can understand when interspecific competition, and more generally the community context, is critical to determine plastic and evolutionary trajectories of species, a question currently remaining largely unclear37. Furthermore, our study allows understanding species’ responses to environmental change. It also extends the partitioning of plastic and genetic components towards a multi-species perspective, a crucially needed step to predict species responses to environmental change38,39.We quantify species’ responses by measuring key traits of all species at the scale of individuals, using highly-resolved and automated video analyses (detailed in ‘Methods’). The measured traits—biomass (i.e. the area of a cell), cell shape (i.e. ratio of the largest to second largest cell size axis of an individual40), and dispersal ability (i.e. gross speed)—are directly linked to ecological, physiological, and behavioral responses, as well as fitness of these species under environmental change29,41. Based on previous studies42,43,44, we expect rapid plastic responses towards lower biomass, cell shape, and dispersal ability. We expect larger contributions of mean trait evolution in the higher salt concentrations as these conditions might express stronger selection. However, competing species may select for different trait values45, and may therefore amplify or impede trait responses to salinity. If a species’ plastic and genetic response to competing species are opposite to its plastic and genetic response to the abiotic environmental change, then initially one might not observe phenotypic change. Only when disentangling the trait change into plastic and genetic components, effects of the abiotic and biotic (here: presence of competing species) environment would be detected.Across all replicates, P. aurelia and S. teres changed phenotypically over the duration of the selection experiment (Fig. 2). The third species, T. thermophila, drastically declined in population density throughout all replicates. In the absence of competition, this density reduction occurred around day 25 for the lowest salinity conditions and around day 20 for the highest salinity conditions. Only for the lowest salinity conditions, low population sizes were maintained throughout the selection phase. In the presence of competition, this density reduction occurred around day 15 for the low salinity conditions and around day 10 for the high salinity conditions. As we did not detect any T. thermophila individuals by the end of the selection phase in the presence of competitors, we assumed this species went extinct in these conditions. Due to the lack of trait data for T. thermophila, we here focus on results found for P. aurelia and S. teres. Interestingly, in the two highest salinity conditions (2 and 4 g/L), S. teres also went extinct (i.e. its population size fell below the detection threshold) in all replicates, except one of the competition treatment in the 4 g/L salt environment. During the selection phase, P. aurelia showed, independent of treatment, a significant reduction in biomass and dispersal ability and a significant increase in cell shape, i.e., individuals became more elongated (Fig. 2a–c). The second species, S. teres, showed a significant increase in biomass, cell shape and dispersal ability (Fig. 2d–f). Importantly, for both species, these generally-observed trait changes were significantly altered by competition and the salinity environment. This was detected by a significant statistical interaction between time and interspecific competition, and between time and salinity (Supplementary Tables S1, S2).Figure 2Temporal phenotypic response of Paramecium aurelia (a–c) and Spirostomum teres (d–f) during the selection phase. Trait values at the start (day 4) and end (day 78) of the selection phase for biomass, quantified as bio-area (a,d), cell shape, quantified as cell size ratio between the major and minor cell axis (b,e), and dispersal ability, quantified as gross speed (c,f) when P. aurelia and S. teres evolved in the absence (blue colors) and presence (orange colors) of competing species along different salinity conditions (indicated by different shades, from light to dark color intensity corresponding to 0, 0.5, 1, 2 and 4 g/l). Dots represent mean trait values of each replicate with error bars showing the standard deviation and representing the phenotypic trait distribution. The slope of the line connecting dots shows the temporal shift in the trait considered. Dots are horizontally jittered for visual aid. Temporal phenotypic differences are shown in Supplementary Figure S1. Supplementary Tables S1 and S2 show the detailed results of the statistical analysis.Specifically, for P. aurelia, the decrease in biomass and dispersal ability was larger for those individuals from the competition treatment (Supplementary Fig. S1). For dispersal ability and cell shape, the effect of competition differed between salinity environments. In the lowest salinity, P. aurelia individuals on average decreased in cell shape during the selection experiment (Fig. 2b). Contrary, P. aurelia individuals in the highest salinity showed an increase in cell shape—individuals became more elongated. For S. teres, individuals from the competition treatment decreased less in biomass on average, but showed a larger increase in cell shape (Supplementary Table S2). However, both of these effects depended on salinity (Supplementary Fig. S1). Competing species had no effect on the temporal response in dispersal ability for S. teres (Supplementary Table S2). Thus, at the end of the selection experiment, the abiotic environmental change and the presence of competing species resulted in a set of different trait values compared to those of the ancestral population.Phenotypic response to salinity and competing species assessed in the common gardenFrom the common garden experiment, we evaluated the effect of exposure to the abiotic salinity environment (historical salinity) and to competing species (historical competition) during the selection phase on the trait values of P. aurelia and S. teres, and wheth
https://www.nature.com/articles/s41598-021-02841-8
Competition alters species’ plastic and genetic response to environmental change