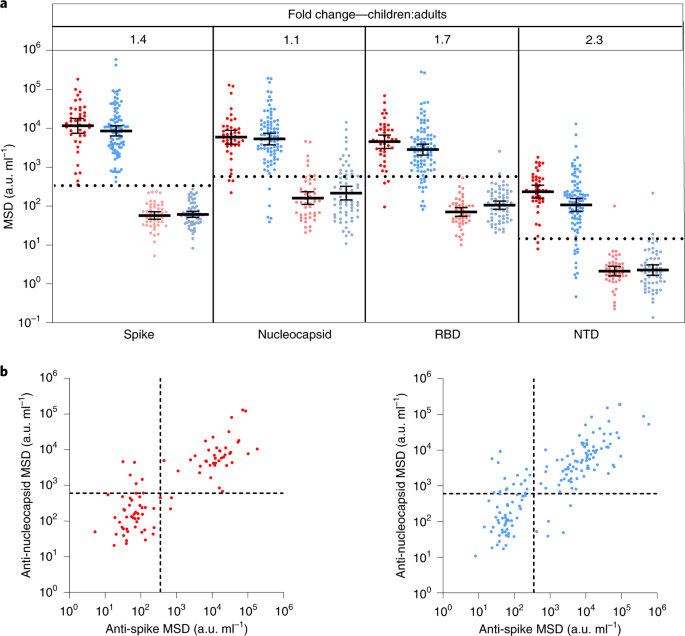
Children develop robust and sustained cross-reactive spike-specific immune responses to SARS-CoV-2 infection Alexander C. Dowell orcid.org/0000-0002-1909-70471, Megan S. Butler1 na1, Elizabeth Jinks1 na1, Gokhan Tut1 na1, Tara Lancaster orcid.org/0000-0002-2872-47941, Panagiota Sylla1, Jusnara Begum1, Rachel Bruton1, Hayden Pearce orcid.org/0000-0002-8380-81221, Kriti Verma1, Nicola Logan2, Grace Tyson orcid.org/0000-0001-9496-56602, Eliska Spalkova1, Sandra Margielewska-Davies1, Graham S. Taylor orcid.org/0000-0002-4807-27971, Eleni Syrimi1, Frances Baawuah3, Joanne Beckmann4, Ifeanyichukwu O. Okike3,5, Shazaad Ahmad6, Joanna Garstang orcid.org/0000-0001-9268-05817,8, Andrew J. Brent9,10, Bernadette Brent9, Georgina Ireland3, Felicity Aiano3, Zahin Amin-Chowdhury3, Samuel Jones3, Ray Borrow orcid.org/0000-0002-0691-656811, Ezra Linley orcid.org/0000-0002-5935-615411, John Wright12, Rafaq Azad12, Dagmar Waiblinger12, Chris Davis2, Emma C. Thomson orcid.org/0000-0003-1482-08892, Massimo Palmarini2, Brian J. Willett orcid.org/0000-0001-8912-32662, Wendy S. Barclay13, John Poh orcid.org/0000-0003-2874-42903, Gayatri Amirthalingam3, Kevin E. Brown3, Mary E. Ramsay orcid.org/0000-0002-7156-76403, Jianmin Zuo orcid.org/0000-0002-8341-465X1, Paul Moss orcid.org/0000-0002-6895-19671 na2 & Shamez Ladhani3,14 na2 Nature Immunology (2021)Cite this article Adaptive immunityInfectious diseasesSARS-CoV-2Viral infection SARS-CoV-2 infection is generally mild or asymptomatic in children but a biological basis for this outcome is unclear. Here we compare antibody and cellular immunity in children (aged 3–11 years) and adults. Antibody responses against spike protein were high in children and seroconversion boosted responses against seasonal Beta-coronaviruses through cross-recognition of the S2 domain. Neutralization of viral variants was comparable between children and adults. Spike-specific T cell responses were more than twice as high in children and were also detected in many seronegative children, indicating pre-existing cross-reactive responses to seasonal coronaviruses. Importantly, children retained antibody and cellular responses 6 months after infection, whereas relative waning occurred in adults. Spike-specific responses were also broadly stable beyond 12 months. Therefore, children generate robust, cross-reactive and sustained immune responses to SARS-CoV-2 with focused specificity for the spike protein. These findings provide insight into the relative clinical protection that occurs in most children and might help to guide the design of pediatric vaccination regimens. The SARS-CoV-2 pandemic has resulted in over 4.2 million deaths so far and the most notable determinant of outcome is age at the time of primary infection1. SARS-CoV-2 infection in children is generally asymptomatic or mild and contrasts with high rates of hospitalization and death in older adults2. As such, there is interest in understanding the profile of the immune response to SARS-CoV-2 in children. Such studies are limited to date but have reported reduced magnitude of both antibody and cellular responses in comparison to adults and an absence of nucleocapsid-specific antibody responses during or early postinfection3,4,5,6. One unique feature of SARS-CoV-2 infection in children is the development of a rare complication known as pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS), also known as multisystem inflammatory syndrome in children (MIS-C), which shares features with Kawasaki disease and toxic shock syndrome7,8. MIS-C develops approximately 2–4 weeks after infection in children with a median age of 9 years9. The immunological basis for this condition is unclear but it is characterized by diffuse endothelial involvement and broad autoantibody production10.One potential determinant of differential immune responses to SARS-CoV-2 across the life course may be the timing of exposure to the four additional endemic human coronaviruses (hCoVs). These comprise the Beta-coronaviruses OC43 and HKU-1, which have 38% and 35% amino acid homology with SARS-CoV-2, and the more distantly related Alpha-coronaviruses NL63 and 229E, each with around 31% homology11. These coronaviruses cause frequent mild childhood infections and antibody seroconversion occurs typically before the age of 5 years. Infection with one of the Alpha- or Beta-coronaviruses provides short-term immunity against reinfection from coronaviruses and represents transient cross-reactive immunity within the subtypes12,13,14. As such, recent hCoV infection might presensitize children against SARS-CoV-2 infection and may explain cross-reactive SARS-CoV-2-neutralizing antibodies in some seronegative children15. Immune responses against hCoV are retained throughout life but do not provide sterilizing immunity13. Consequently, recurrent infections are common, generating concern that a similar pattern will be observed after SARS-CoV-2 infection.COVID-19 vaccines are now being administered widely to adult populations and are also being delivered to children in some countries. Therefore, it is imperative to understand the profile of SARS-CoV-2-specific immune responses in children after natural infection to inform vaccination strategy. In this study, we provide a comprehensive characterization of the convalescent humoral and cellular immune response in a cohort of 91 primary school-aged children compared with 154 adults taking part in the COVID-19 surveillance in school KIDs (sKIDs) study16. We demonstrate a markedly different profile of immune response after SARS-CoV-2 infection in children compared to adults. These findings have potential implications for understanding protective or pathological immune responses to infection in children and might help to guide and interpret COVID-19 vaccination regimens for children.Blood samples were obtained from 91 children and 154 adults, including 35 children and 81 adults known to be seropositive in previous rounds of testing. All infections were asymptomatic or mild and no staff or students in the cohort required medical care or hospitalization. The median age of children was 7 years (range 3–11) while that of adults was 41 years (range 20–71). The SARS-CoV-2 antibody profile was assessed using the Meso Scale Discovery (MSD) V-PLEX serology platform to determine serological responses against spike, receptor binding domain (RBD), N-terminal domain (NTD) and nucleocapsid (N). In total, 47% of children and 59% of adults were seropositive (Supplementary Table 1). To ensure the sensitivity of our assays, we obtained convalescent plasma samples from 35 children with PCR-confirmed SARS-CoV-2. Thirty-four were seropositive in the assay while 1 donor mounted no detectable antibody response to any antigen tested. Prepandemic plasma samples from 9 children and 50 adults all gave negative results and demonstrated the specificity of the assay (Extended Data Fig. 1).Seropositive children and adults demonstrated broadly similar antibody responses against viral proteins. However, geometric mean antibody titers against all four regions were higher in children, most notably against the NTD and RBD domains, which showed 2.3-fold and 1.7-fold increases, respectively, although these did not reach statistical significance (Fig. 1a). In contrast to previous reports3, we also observed antibody responses against nucleoprotein, with a 1.3-fold increased antibody titer compared to adults (Fig. 1a,b).Fig. 1: Children and adults develop coordinated antibody responses to SARS-CoV-2.a, SARS-CoV-2 antibody levels measured by MSD assay in children (n = 91) and adults (n = 154). Serostatus was assigned based on spike serology and used to divide the cohorts into seropositive (red/blue) and seronegative (light red/light blue) (seropositive/negative children n = 43/48, adults n = 91/63, respectively). The dotted lines represent cutoff values for serostatus. Fold change indicates the difference between the GMTs in seropositive children and adults. The bars indicate the geometric mean with 95% confidence interval (CI). b, The level of the spike- and nucleocapsid-specific antibody response was correlated within individual donors and revealed a coordinated response to both proteins. a.u., arbitrary unit.Source dataSARS-CoV-2 infection boosts hCoV binding antibodies in childrenPre-existing immune responses against seasonal coronaviruses might act to modulate clinical outcome after primary SARS-CoV-2 infection and cross-reactive neutralizing antibodies have been reported in SARS-CoV-2-seronegative children15. Consequently, we compared antibody levels against the four hCoVs in SARS-CoV-2 in seronegative and seropositive children and adults.A 1.2–1.4-fold increase in the titer against hCoV was evident in SARS-CoV-2 seropositive adults compared to the seronegative group. In contrast, antibody levels against all 4 viruses were boosted markedly in SARS-CoV-2 seropositive children, with 2.3, 1.9, 1.5 and 2.1-fold higher antibody levels compared to the seronegative group. These were significant for OC43 and HKU-1 (P = 0.0071 and P = 0.0024 Brown–Forsythe and Welch’s analysis of variance (ANOVA), with Dunnett’s T3 multiple comparison test) (Fig. 2a). Notably, the level of hCoV-specific antibodies in seropositive children was comparable to adults, whereas seronegative children possessed lower responses than adults (Supplementary Table 1).Fig. 2: Antibody responses to hCoVs are back-boosted by SARS-CoV-2 in children.a,b, Antibody titers to the seasonal hCoV coronaviruses (a) and other respiratory viruses (b) in children (red) and adults (blue) based on SARS-CoV-2 serostatus (dark, seropositive, light, seronegative; seropositive/negative children n = 43/48, adults n = 91/63, respectively). Fold change indicates the difference between the GMTs in seropositive children and adults. The bars indicate the geometric mean with the 95% CI. Only significant differences are shown. Brown–Forsythe and Welch’s ANOVA with Dunnett’s T3 multiple comparison tests were used.Source dataTo assess if this effect was specific to hCoV or represented a more general effect of SARS-CoV-2 infection on antibody responses against heterologous infection, we also examined antibody titers against influenza subtypes and respiratory syncytial virus in relation to SARS-CoV-2 serostatus. No change in antibody titer against these viruses was seen in either children or adults (Fig. 2b). These data show that SARS-CoV-2 infection in children specifically boosts humoral responses against hCoV.SARS-CoV-2 antibodies in children cross-react with Beta-hCoVGiven the increase in hCoV-specific antibody titers after SARS-CoV-2 infection in children, we next assessed to what extent this was cross-reactive against SARS-CoV-2 or could represent an hCoV-specific response. As such, recombinant S1 or S2 domain protein from SARS-CoV-2 was used to preabsorb plasma samples before assessment of antibody levels to both SARS-CoV-2 and the four hCoV subtypes.As expected, preabsorption with both the S1 and S2 domains markedly reduced antibody titers against total spike (P < 0.0001 and P = 0.0024, respectively, Friedman test with Dunn's multiple comparisons test). The S1 domain, but not the S2 domain, absorbed RBD- and NTD-specific antibodies against SARS-CoV-2 while no influence was observed in relation to nucleocapsid-specific binding for either domain (Fig. 3). Of note, the S1 domain did not reduce antibody binding to any of the four hCoV subtypes, indicating little evidence for cross-reactive antibodies against this domain. The S2 domain, however, selectively reduced antibody binding to the two hCoV Beta-coronaviruses OC43 and HKU-1 (P < 0.0001 and P = 0.0014, respectively by one-way repeated measures ANOVA with Holm–Sidak's multiple comparison test). No such effect was observed in relation to binding to the Alpha-coronaviruses NL63 and 229e (Fig. 3).Fig. 3: SARS-CoV-2 S2 domain antibodies cross-react with hCoV.Plasma from SARS-CoV-2 seropositive children (n = 21) was assessed for binding to the spike protein of the 4 hCoVs or the spike or nucleocapsid regions of SARS-CoV-2. Plasma was either applied neat (control) or after preabsorption with either recombinant spike S1 domain (spike 1 block) or spike S2 domain (spike 2 block). S1 preabsorption markedly reduced binding to SARS-CoV-2 spike with no effect on hCoV, while S2 preabsorption reduced binding to OC43 and HKU-1. One-way repeated measures ANOVA with Holm–Sidak's multiple comparison test or Friedman test with Dunn's multiple comparisons test were used as appropriate.Source dataThese data show that the S1 region is the immunodominant target of antibody responses in children. However, antibodies that are cross-reactive against Beta-coronavirus are largely specific for the S2 domain and contribute to the higher SARS-CoV-2-specific titer in children.Children develop robust cellular immune responses to spike proteinWe next assessed the magnitude and profile of the cellular immune response against SARS-CoV-2 in children and adults. Enzyme-linked immunosorbent spot (ELISpot) analysis against overlapping peptide pools from spike and a combination of nucleocapsid and membrane and envelope (nucleoprotein and viral membrane (N/M)) was performed on samples from 57 children and 93 adults, including 37 and 64 respectively who were seropositive.As expected, ELISpot responses were common in SARS-CoV-2 seropositive donors with 89% (33 out of 37) of seropositive children and 80% (51 out of 64) of seropositive adults showing a positive ELISpot response to spike and/or the N/M pool.The magnitude of the cellular response against spike was 2.1-fold higher in children, with median values of 533 spots per million compared to 195 in adults (P = 0.0003, Brown–Forsythe and Welch's ANOVA with Dunnett's T3 multiple comparisons test) (Fig. 4a). Cellular responses against the N/M pool were relatively lower in children compared to spike such that the S:N/M ratio was markedly elevated in children at 4.7 compared to 1.8 in adults (P = 0.0007, Brown–Forsythe and Welch's ANOVA with Dunnett's T3 multiple comparisons test) (Fig. 4b). Eighty-six percent of children showed a positive response to spike while only 43% responded to the N/M pool. Within adults these values were 70% and 63%, respectively (Fig. 4c).Fig. 4: Spike-specific T cell responses in SARS-CoV-2 seropositive and seronegative children.a, SARS-CoV-2-specific T cell responses in children (n = 57, red) and adults (n = 83, blue) based on SARS-CoV-2 serostatus (dark; seropositive, light; seronegative). SARS-CoV-2 serostatus was 37/20 seropositive or negative in children and 64/29 seropositive or negative in adults, respectively. The assay used IFN-γ ELISpot using pepmixes containing overlapping peptides to spike, N/M or influenza and is shown in relation to serostatus. b, The magnitude of the spike-specific cellular response was compared to that against N/M and displayed as a ratio in seropositive and seronegative adults and children, as indicated. The bars indicate the mean. Brown–Forsythe and Welch's ANOVA with Dunnett's T3 multiple comparisons tests were used. c, Proportions of individuals within each cohort who demonstrated a cellular response to S or N/M peptides from SARS-CoV-2. d, Cytokine concentration within supernatants from the ELISpot cultures (n = 12 children, red; n = 8 adults, blue). The bars indicate the mean ± s.d. e, hCoV-specific cellular responses showed equivalent expansion after stimulation of PBMCs from SARS-CoV-2 seronegative children with the SARS-CoV-2 S2 domain pepmix (n = 11). Cultures were stimulated for 9 d and then assessed by IFN-γ ELISpot to the pepmix of the S2 domain from SARS-CoV-2 or the Alpha (OC43 and HKU-1) or Beta (NL63 and 229E) hCoV. Expansion is shown relative to unstimulat
https://www.nature.com/articles/s41590-021-01089-8
Children develop robust and sustained cross-reactive spike-specific immune responses to SARS-CoV-2 infection