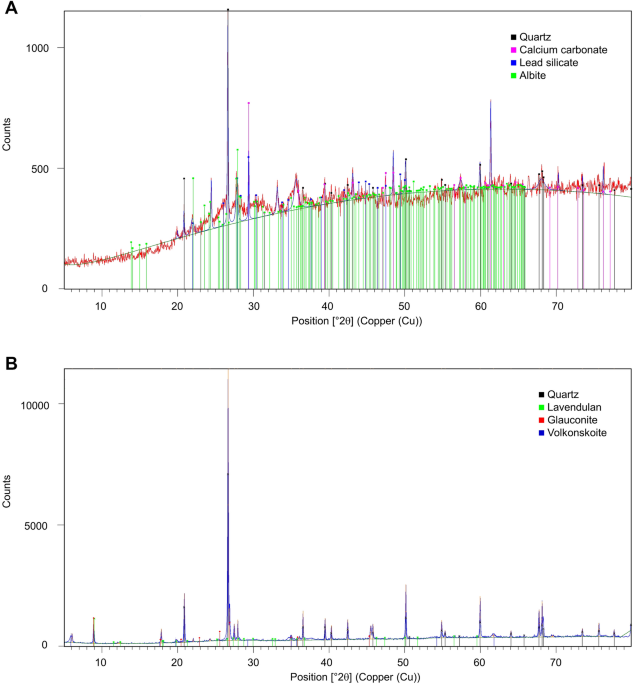
Characteristics and impact of aged coal ash with slag emplaced in a karst cave: the case of Divaška jama, Slovenia A mixture of coal bottom ash and slag, with a fraction of fly ash (CAFAS) from steam locomotives, was placed in the cave Divaška jama to delimit and level tourist trails. Emplacement began in 1914 and carried on for several decades. The CAFAS mixed with other cave material gradually changed its structure and appearance. Currently the concentration of some elements in the CAFAS (As, Cu, Hg, Ni, Pb, Zn), and also to a lesser extent in cave sediments (Cr, Cu, Ni), indicates a possibly harmful effect on sediment-associated biota based on ecotoxicological assays. Compared to the cave sediment, the CAFAS contains distinctly different mineral phases and presents a different source of radioactivity. Microbial metabolic activity of CAFAS is low, 0.22 μl O2/gDW h, but higher than that of cave sediment. The present environmental hazards from CAFAS are estimated to be low. Whereas the emplacement of CAFAS was seen initially a long-term solution for waste disposal and management of the cave, it turned out that CAFAS enriches the underground environment with inorganic and organic compounds and disperses pollution into the cave ecosystem. After its removal from the cave, the CAFAS should be investigated thoroughly due to its susceptibility to alteration. Combustion of coal produces gas emissions and solid by-products in the form of fly ash, bottom ash and boiler slag. Coal ash (CA), especially bottom ash from steam power generation, was one of the first massively produced wastes related to the Industrial Revolution. CA contains Al2O3, CaO, Fe2O3, K2O, MgO, Na2O and SiO2 as its major mineral components1; others are present in small quantities, depending largely upon the properties of each individual coal2. Polycyclic aromatic hydrocarbons (PAHs) from unburned or incompletely burned organic matter remain within the coal ash3. Most of the CAs, once considered harmless and hence deposited widely in landscapes, landfills and mines, are now the subject of controversial discussions about the hazards they might present4. Residual coal dust and combustion by-products within the ashes have a genotoxic and mutagenic effect on biota, related to the release of PAHs and heavy metals5. When stored in settling ponds in the immediate vicinity of waterways, CA poses a considerable risk to both surface water and groundwater6. It is estimated that more than 9000 ash ponds are in operation worldwide7. Technical failure of such ponds can have catastrophic consequences, as in the case of the Kingston power plant in Tennessee, USA, in 20088 or in the case of Sutton Lake, USA, where uncontrolled CA spills occurred from impoundments and landfills in flood-prone areas9. In a study eight years after the spill, CA was found to be buried beneath natural sediment (13–18 cm) in a nearby creek10. High concentrations of ash, metals, and metalloids were still present in the shallow sediments (0–3 cm) near the Kingston site, indicating redistribution of CA from upstream. It was also found that metals and metalloids associated with CA were not mobilized by river water. Previous studies have shown that certain conditions in water and river sediments can control the mobilization of certain pollutants from coal combustion residues. For example, As and Se are mobilized under reducing and oxidizing conditions, respectively. Low pH affects the leachability of metals and high pH of metalloids; ORP and pH together determine the speciation and mobility of elements from coal combustion residues11. It is well known that contamination of water supplies by CA poses a physical threat to animal and human life12,13. Research and case studies have highlighted the harmful long-term effects of bioaccumulation of metalloids in the ecosystem14, particularly selenium, which bioaccumulates in aquatic animals and plants at the base of the food chain15.Historically the increasing production of CAs soon became a problem, not only near industrial centres but also along the railway lines where they were dropped from steam locomotives. Most of the ejected material consisted of coal bottom ash and slag and a part of fly ash. To remove part of this waste from the landscape, in 1914 some material was introduced into a karst cave at Divača, Slovenia, where it was used to help develop tourist trails16. It was deposited, mostly along the trails, on top of slippery natural clay. During the decades that followed, about 100 m3 of this waste was brought into the cave. By mixing with cave sediments, debris and infiltrates at high humidity, coal bottom ash and slag with a fraction of fly ash gradually turned into blackish dirt (hereafter CAFAS). Hardly any data relating to the long-term effects of CAFAS on groundwater, karst caves, karst ecosystems and accompanying biota are available. Some studies of negative impacts upon local ecosystems, ranging from the disruption of natural drainage systems to the absence of vegetation due to nutrient deficiency, poor soil structure, acidity and metal toxicity, have been conducted at abandoned mines in various countries17,18,19.The current study addressed the ongoing leaching behaviour of CAFAS, unique material, from Divaška jama, and its toxicity and microbial activity compared to natural cave sediment. The results are important to aid estimation of the long-term effects of allochthonous CAFAS waste on groundwater and the underground ecosystem when deposited under stable environmental conditions of high humidity and low temperature, and also to advise planning of management alternatives.CAFAS introduced into Divaška jama has changed the appearance and natural conditions of the cave (Fig. 2). To estimate its current impact on the cave, CAFAS was compared with sediment derived from eroded bedrock, deposited in the cave during the Pleistocene. Although the two samples did not have the same origin, some elements in both showed similar concentrations, e.g. Al, Be, Cd, Fe, Mg, V; some were comparatively higher within the CAFAS (Ca, Cu, Na, Pb, As, Hg, Zn), and some were higher within the sediment (Ni, Mn, K, Co, Cr) (Table 1). Identified crystalline phases between natural cave sediment and CAFAS were distinctively different (Table 2). In comparison to cave sediment, the CAFAS contained a large proportion of non-crystalline amorphous phase material (Fig. 1). Chemical and biological changes occurred in the CAFAS after its introduction into the cave; in particular it became enriched in organic matter. The exact physico-chemical changes of CAFAS cannot be determined because the original material introduced into the cave is not available for analysis. In general, coal ash does not contain organic material, and if it does, it is usually unburned or incompletely burned organic material, which usually constitutes a very small fraction20. CA and bottom ash are formed in a combustion chamber at high temperatures and under dry and oxygen-rich conditions. When the material is exposed to the external environment, chemical changes occur as it reacts with water, which is in vapour or liquid form. This results in new mineral phases and leachate, which usually contains soluble salts, mobilised metals, and other toxic pollutants15. The CAFAS analysed in this study contained a significant amount of elements (C, N, P) that are critical for microbial metabolism21 and can be attributed to increased microbial activity in CAFAS compared to cave sediment (Table 4).Table 1 Physico-chemical and radioactivity analyses of cave sediment and CAFAS from Divaška jama.Table 2 Identified crystalline phases of cave samples.Figure 1Diffractograms of CAFAS (A) and cave sediment (B).The total element concentration is insufficient to estimate the environmental burden in the sediments, but the addition of data on the bioavailability, toxicity and mobility of the elements provides a more comprehensive overview24,25. First, the results of chemical analyses were compared with a consensus-based sediment quality guideline that defines a threshold effect concentration (TEC), below which adverse effects on freshwater sediment-dwelling organisms are unlikely23. Because some of the values exceeded TEC and because CAFAS is to be extracted completely from the cave and further managed, these values were compared with the current legislation to examine options for waste management. Neither CAFAS nor-even cave sediment would be a suitable soil treatment in agriculture (Table 1). Due to the origin of CAFAS, i.e. coal ash and slag, it can be classified as non-hazardous waste that should be tested for leachate behaviour and then landfilled according to legislation (Decree on waste landfill, Uradni list RS, 2014, 10/14). Considering growing environmental concerns and the limited space available for landfilling, a more attractive alternative to the disposal of such no-value waste might be recycling, with onward use in various geotechnical applications, for example as geotechnical composites26. Elevated concentrations of natural radionuclides are, however, commonly found in by-products of coal combustion27. In this study, both cave sediment and CAFAS expressed comparable low levels of radiation, but they differed in the radioactivity of natural radionuclides (Table 1). Even if CAFAS fits the legislation criteria on specific gamma irradiation for application in construction materials (Decree on limit doses, reference levels and radioactive contamination, Uradni list RS, 2018, 18/18; Decree on radiation activities, Uradni list RS, 2018, 19/18), it should still be investigated thoroughly because of its long exposure under cave conditions.The concentrations of elements in the material provide crucial information for considerations of proper waste management. As mentioned above, the mobility and leaching of major and trace elements are additional important characteristics to help assess the potential impact on the environment and biota correctly. To simulate the effects of leaching and environmental changes with respect to the altered chemistry of infiltrating water, two different pH values were used. Greater quantities of ionic compounds were leached from CAFAS as indicated by higher electrical conductivity values. In both cases the eluates were equilibrated at the end of the experiment at a pH around neutral (Table 3). The pH influences the leaching behaviour of the toxic compounds in CA28. The highest leaching rate was at pH 5.21, when 0.205% Cu was mobilized from the cave sediment and 0.154% Ni from the CAFAS (Table 3). Due to the low mobility of the compounds investigated, and because the concentrations of the readily soluble fractions were generally below 0.5%, the potential environmental hazard and threat for biota and groundwater is estimated to be low. This is in line with previous findings, which show that the pollution potential of aged ash is significantly reduced compared to that of fresh and recently deposited ash29. It is also not expected that the current level of leaching of residual compounds from CAFAS (Table 3) will have additional negative impacts on the cave ecosystem. On the other hand, it has contributed to an unaesthetic and unnatural appearance of the cave (Fig. 2).Table 3 Characteristics of water leachate and eluates with the percentage of compounds leached from cave sediment and CAFAS.Figure 2Removal of deposited CAFAS on tourist footpath in Divaška jama, 2019 (Photo: courtesy by Borut Lozej).The quantity of compounds leached over the decades, and the cumulative impact, cannot be assessed properly because no unaltered sample of the original material is available for comparison. It is possible that CAFAS had strong negative impacts on biota and groundwater prior to its transformation to its current state, but this has not been documented due to the lack of monitoring in the past. It is worth noting that the mobility of compounds in combustion waste is strongly dependent upon coal properties, combustion technology and the behaviour of trace elements during combustion2. The amount of the mobilised fraction previously leached-out in the cave environment thus remains unquantifiable. As long as CAFAS is present on site, however, leaching will continue, especially of compounds whose concentrations remain high and which have demonstrably been mobilised, e.g. copper compounds (Table 3).Microbial activity and toxicityCAFAS and cave sediment contained comparable concentrations of total and cultivable microbial biomass on tested microbiological media (Table 4), but the CAFAS contains higher levels of macronutrients, especially C, but also N and P (Table 1). This can be attributed to the gradual enrichment of the CAFAS with organic debris, infiltrates, and cave sediments, making it an enriched substrate for microbial metabolism. Whereas the cave sediments displayed zero ETSA (electron transport system activity), the CAFAS activity was measured as 0.22 μl O2/gDW h. This is close to the lowest value recorded for sediments from the comparable hyporheic zone, ranging from 0.3 to 28.9 μl O2/gDW h, based on an allochthonous source of organic matter30. These results are consistent with those of other studies, indicating that the organic matter content of sediments is one of the main drivers of microbial metabolic activity30,31,32,33. Interestingly, in a previous study34 the highest toxic effect of Cu in sediments upon microbial activity was expressed at far lower concentrations than those of Cd, Pb and Zn. The Cu eliminated ETSA completely, whereas the other three metals reduced activity by 40–45%. In contrast, the toxic effects of metals such as Cu can be reduced by high concentrations of nutrients or cells, and those of Cd are reduced by clay minerals35. Specific properties of cave sediment and CAFAS have different effects on the metabolic activity of microbial populations. CAFAS can be considered active metabolically and, due to its quantity, it is an important part of the cave ecosystem. CAFAS with its transformation became an important microbial habitat in Divaška jama over time due to the physical cave environment, e.g. the presence of dripping water, autochthonous cave sediments, organic debris and infiltrates at high humidity, and the associated microbial activity.Table 4 Microbial biomass and electron transport system activity (ETSA).In the ecotoxicity assays, both the cave sediment and the CAFAS exhibited toxicity towards the bacterial indicators A. fischeri and E. coli (Table 5), which are used commonly in similar environmental studies36,37. Interestingly, among the E. coli strains tested, the laboratory strain DH5α was more resilient than the E. coli isolated from nature. Nevertheless, these ecotoxicological assays do not identify the exact inhibition factor(s) and mechanisms of toxicity, which range from destabilisation of macromolecules through the exchange of essential cofactors, to the blocking of essential functional groups and the production of reactive oxygen species (ROS)38. Metal-induced toxicity is commonly attributed to oxidative stress39,40,41,42,43,44,45; in the current study this could be related to elevated concentrations of As, Cr, Cu, Hg, Ni and Pb (Table 1).Table 5 Assessing toxicity of solid samples based on A. fischeri and eluates based on E. coli indicator systems.Dripping water introduces new biomass and organic matter into caves46,47,48 and as such can influence microbial activity and leaching when hitting cave sediments. For example, whereas the alkaline pH of dripping water reduces the solubility of Fe, Mn and Mg, it increases the solubility of Al49, which represents the major metal component in both the CAFAS and the cave sediment samples (Table 1). Microbial processes su
https://www.nature.com/articles/s41598-021-02842-7
Characteristics and impact of aged coal ash with slag emplaced in a karst cave: the case of Divaška jama, Slovenia