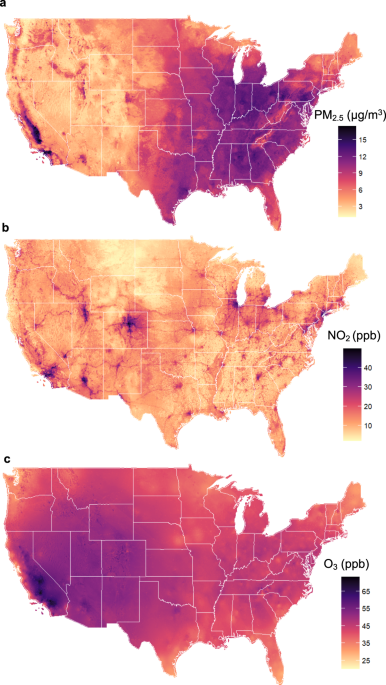
A national cohort study (2000–2018) of long-term air pollution exposure and incident dementia in older adults in the United States Liuhua Shi orcid.org/0000-0001-8165-46441 na1, Kyle Steenland1 na1, Haomin Li2, Pengfei Liu3, Yuhan Zhang2, Robert H. Lyles4, Weeberb J. Requia orcid.org/0000-0002-7564-33645, Sindana D. Ilango6, Howard H. Chang1,4, Thomas Wingo orcid.org/0000-0002-7679-62827, Rodney J. Weber3 & Joel Schwartz orcid.org/0000-0001-6168-378X8 Nature Communications 12, Article number: 6754 (2021) Cite this article Alzheimer’s diseaseEnvironmental impact Air pollution may increase risk of Alzheimer’s disease and related dementias (ADRD) in the U.S., but the extent of this relationship is unclear. Here, we constructed two national U.S. population-based cohorts of those aged ≥65 from the Medicare Chronic Conditions Warehouse (2000–2018), combined with high-resolution air pollution datasets, to investigate the association of long-term exposure to ambient fine particulate matter (PM2.5), nitrogen dioxide (NO2), and ozone (O3) with dementia and AD incidence, respectively. We identified ~2.0 million incident dementia cases (N = 12,233,371; dementia cohort) and ~0.8 million incident AD cases (N = 12,456,447; AD cohort). Per interquartile range (IQR) increase in the 5-year average PM2.5 (3.2 µg/m3), NO2 (11.6 ppb), and warm-season O3 (5.3 ppb) over the past 5 years prior to diagnosis, the hazard ratios (HRs) were 1.060 (95% confidence interval [CI]: 1.054, 1.066), 1.019 (95% CI: 1.012, 1.026), and 0.990 (95% CI: 0.987, 0.993) for incident dementias, and 1.078 (95% CI: 1.070, 1.086), 1.031 (95% CI: 1.023, 1.039), and 0.982 (95%CI: 0.977, 0.986) for incident AD, respectively, for the three pollutants. For both outcomes, concentration-response relationships for PM2.5 and NO2 were approximately linear. Our study suggests that exposures to PM2.5 and NO2 are associated with incidence of dementia and AD. Dementia is a major public health issue, affecting >47 million people worldwide1. Alzheimer’s disease (AD) contributes to about two-thirds of dementia cases and is the sixth leading cause of death in the United States2. In response, the National Alzheimer’s Project Act was signed into law to overcome dementia, and the National Plan was launched with Goal 1 aiming to prevent and effectively treat dementia (delay onset, slow progression) by 20253. As there are no disease-modifying treatments for the most common types of dementia, it is a top research priority to identify modifiable risk factors for dementia that can be intervened on at the population level.There is growing evidence associating air pollution with neurodegenerative disease. A systematic review by Peters et al.4 found nine longitudinal studies of air pollution and AD and related dementias (ADRD). Among them, five of six showed a positive association between increased exposure to PM2.5 and dementia or AD; four of four showed an association between NO2 and dementia or AD, whereas one of three did so for ozone (O3). Fu and Yung5 published a review and meta-analysis of AD and air pollution, and found a twofold excess risk of AD for a 10 µg/m3 increase of PM2.5 among six studies, and no increased risk for NO2 in four studies, nor for O3 in three studies. There have been several longitudinal studies since these reviews, with the majority finding positive associations between air pollutants and either dementia or AD6,7,8,9,10,11,12,13,14. A few of these studies examine the associations in US populations, and these studies have almost exclusively used hospitalization as a measure of morbidity6,7,11,13. The diagnosis of ADRD, however, likely occurs in doctor visits, and ADRD does not generally result in hospitalizations. Thus, hospitalization records may not well represent either disease incidence or prevalence in the overall population, and likely leads to an underestimation of the number of cases, and unclear generalizability. In addition, neuropathologic changes are known to occur many years prior to the diagnosis15, and the relevant time window in which air pollution might increase the risk of dementia or AD is unclear.To address these knowledge gaps in studying ADRD incidence in the US, here we constructed a national, population-based cohort study from Medicare data to investigate the impact of long-term exposure to PM2.5, NO2, and warm-season (May to October) O3 on dementia and AD incidence. To better measure disease incidence, we required a 5-year ‘clean’ period without events of interest after enrollment in Medicare system and used all Medicare claims nationwide (2000–2018), including inpatient and outpatient claims, carrier file (primarily doctor visits), skilled nursing facility, and home health-care claims to identify the first diagnosis of ADRD. We used high-resolution (1 km × 1 km) daily surface-level concentration fields of PM2.5, NO2, and O3 for 2000–2016, estimated based on ground observations, satellite data, chemical transport modeling, land use, and meteorological data using national spatiotemporal ensemble exposure models16,17,18. We assigned air pollution exposure to subjects based on resident ZIP code, and calculated time-varying 5-year lagged moving averages for each follow-up year.Table 1 provides descriptive information on the dementia cohort and AD cohort. Both cohorts were followed after requiring a 5-year period without events of interest to better capture disease incidence. There were 12.2 and 12.4 million people in dementia and AD cohorts, respectively (Table 1). Most of the studied subjects (78.5% and 78.1% for dementia and AD, respectively) entered the cohorts between ages 65 and 74. The median follow-up was 7 years in both cohorts. More than 90% were not eligible for Medicaid, indicating that most were defined as being above the poverty level19. A majority of the study population had comorbidity at some point during follow-up. 16.6% developed dementia (~2.0 million cases), 6.5% developed AD (~0.8 million cases), and Supplementary Table 1 presents detailed demographic information for the cases and non-cases.Table 1 Descriptive statistics for the study population.Air pollution levelsThe average annual level of PM2.5 of cohort participants during the study period, 9.3 µg/m3, was below the US EPA standard of 12 µg/m3; The average NO2 level was 17.1 ppb, considerably below the EPA annual standard of NO2 of 53 ppb. The annual warm-season average O3 was 42.6 ppb. EPA does not have a standard for annual warm-season O3. As a reference, the EPA standard for daily maximum of 8-hour average O3 is 70 ppb (Table 1). We examined warm-season O3, because O3 is more readily formed in the warm season20, and this metric is often used in long-term epidemiological studies21. Fig. 1 shows the distribution of the three pollutants across the US during our study period, as estimated by the exposure models used in our analysis. PM2.5 is highest in the eastern US and in California, O3 in the West, and NO2 (largely produced by traffic) in urban centers. Further detail on exposure levels can be found in Supplementary Table 2. The three pollutants in our data were only modestly correlated. The Pearson correlations between pollutants (average exposure within the past 5 years) were as follows: PM2.5 and O3 0.22, NO2 and O3 0.19, and NO2 and PM2.5 0.39.Fig. 1: Maps of the spatial distributions of air pollutants studied.The three panels present the average concentrations of a annual PM2.5 (μg/m³)16, b annual NO2 (ppb)17, and c warm-season O3 (ppb)18 at 1-km2 resolution across the contiguous United States over the study period, respectively. Map was made from the census bureau shapefile (cb_2017_us_county_500k.shp, https://www2.census.gov/geo/tiger/GENZ2017/shp/) using R software, and no licenses are required as this map was provided free of any copyright restrictions. Source data are provided as a Source Data file.Health effect estimatesFig. 2 provides the main study results from the Cox proportional hazards models stratified by individual characteristics, adjusting for neighborhood-level socioeconomic status (SES) (see details in Methods), behavioral risk factors, health-care capacity variables, and residual temporal and spatial trends (see Methods). An interquartile range (IQR) increase in the 5-year average of the annual PM2.5 (3.2 µg/m3) in the 5 years prior to diagnosis was associated with an increased risk of dementia (HR = 1.061, 95% CI: 1.056, 1.067) in single-pollutant models, which changes little in models with other pollutants. An IQR increase in 5-year average NO2 (11.6 ppb) is associated with an HR of 1.035 (95% CI: 1.028, 1.042) in single-pollutant models, dropping to 1.019 (95% CI: 1.012, 1.026) in multi-pollutant models. An IQR increase in the 5-year average of warm-season O3 (5.3 ppb) has little effect on dementia rates, with HRs of 1.002 (95% CI: 0.998, 1.005) in single-pollutant models and 0.990 (95% CI: 0.987, 0.993) in multi-pollutant models.Fig. 2: Results of the Cox proportional hazards models.The two panels present the hazard ratios of a dementia (n = 12,233,371 individuals examined) or b Alzheimer’s disease (AD, n = 12,456,447 individuals examined) associated with per IQR increase in annual PM2.5, or annual NO2, or warm-season O3 concentration, respectively. The estimated hazard ratios were obtained using single pollutant, bi-pollutant, and tri-pollutant models. Error bars stand for the 95% confidence intervals. The gray and white stripes are used to distinguish any two adjacent models. Source data are provided as a Source Data file.The findings for AD have a similar pattern to those for dementia, but the hazard ratios (HRs) are higher per IQR increase, being 1.078 (95% CI: 1.071, 1.086) for PM2.5, 1.050 (95% CI: 1.042, 1.059) for NO2, and 0.999 (95% CI: 0.995, 1.003) for O3 assessing each pollutant individually. After adjusting for co-pollutants, the effect estimates were similar for PM2.5 (HR = 1.078, 95% CI: 1.070, 1.086) and attenuated for NO2 (HR = 1.031, 95% CI: 1.023, 1.039), while O3 is slightly protective (HR = 0.982, 95% CI: 0.977, 0.987).Concentration–response relationshipsFigure 3 presents penalized spline curves for the three pollutants, derived from the tri-pollutant models. The concentration–response (C-R) relationships for PM2.5 are approximately linear for both dementia and AD across the exposure distribution, although for AD there is a suggestion of a steeper slope below 8 µg/m3. For NO2, the C-R curves for dementia and AD are linear for low concentrations (<25 ppb), and then level off for higher concentrations. The curves for O3 are essentially flat for both endpoints until high, and rarely occurring concentrations. These results suggest that the adverse effects of PM2.5 and NO2 on dementia or AD are at least retained, if not strengthened, at low levels of air pollution exposure (e.g., below the WHO air quality guidelines: PM2.5 ≤ 10 μg/m3, NO2 ≤ 20 ppb). Across the 0.5th to 99.5th percentile of the exposure distribution, PM2.5 shows the strongest effect on dementia or AD among all pollutants.Fig. 3: Concentration-response curves. Panel.a presents the probability distribution functions (PDF) of long-term PM2.5, NO2, and O3 exposures; Panel b presents the concentration–response relationships between each pollutant and dementia; Panel c presents the concentration-response relationships between each pollutant and Alzheimer's disease (AD). The concentration–response curves, derived from the tri-pollutant models, are shown for the concentration ranges between 0.5th to 99.5th percentiles of the pollutants, i.e. with 1% poorly constrained extreme values excluded. Shading areas (from the darkest to the lightest) in a represent pollutant concentration ranges of the IQR (i.e., 25th to 75th percentiles), 95% (2.5th to 97.5th), and 99% (0.5th to 99.5th), respectively. Source data are provided as a Source Data file.Effect modificationsWe examined five potential effect modifiers (sex, race (white, Black, other), Medicaid eligibility, urbanicity (expressed in quartiles of population density), and age (<75, ≥75). Fig. 4 shows HRs in each subgroup, based on the interaction term between exposure and the potential effect modifier. Most marked results were seen for an increased hazard of dementia and AD for Black individuals compared with white individuals in relation to both PM2.5 and NO2; a similar pattern was found for those eligible for Medicaid. At the same time, those living in the rural areas (i.e., lowest quartile of population density) were found to have notably lower effect estimates between both dementia and AD and both PM2.5 and NO2. Regarding age, those <75 had a markedly stronger association between dementia and both PM2.5 and NO2, while the association was stronger between AD and both PM2.5 and NO2 among those older than 75. Finally, we found little evidence of an interaction between PM2.5 or NO2, and sex in relation to dementia or AD. For O3, all subgroup-specific estimated HRs were below one, and the association with both endpoints was stronger among those not eligible for Medicaid or those living in rural areas. The p values for testing the null hypothesis that the estimated associations are the same between subgroups classified by a subpopulation indicator are shown in Supplementary Table 3.Fig. 4: Effect modifications by sex, race, Medicaid eligibility, age, and population density.Results represent the hazard ratios of dementia (n = 12,233,371 individuals examined) or Alzheimer's disease (AD, n = 12,456,447 individuals examined), from the tri-pollutant models, per IQR increase in 5-year average PM2.5, NO2, or O3. Error bars stand for the 95% confidence intervals. The blue dashed lines indicate the overall effect estimates for all groups. 'Other' includes Asian, Hispanic, American Indian or Alaskan Native, and unknown. 'Density Q1–Q4' denotes quartiles of population density, i.e., low population density, low-medium population density, medium-high population density, and high population density. Source data are provided as a Source Data file.Sensitivity analysisAssociations between long-term exposure to PM2.5, NO2, O3, and dementia or AD were robust to a series of sensitivity analyses. First, a more strict 'clean period' by excluding anyone who had a diagnosis for dementia or AD in their first 10 years of follow-up yielded results similar to the main analyses (Supplementary Table
https://www.nature.com/articles/s41467-021-27049-2
A national cohort study (2000–2018) of long-term air pollution exposure and incident dementia in older adults in the United States