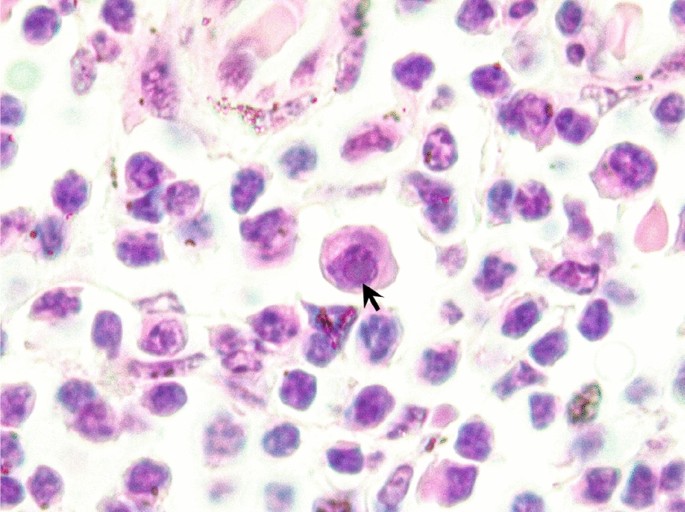
1.Cassens, I. et al. Independent adaptation to riverine habitats allowed survival of ancient cetacean lineages. Proc. Natl. Acad. Sci. 97, 11343–11347. https://doi.org/10.1073/pnas.97.21.11343 (2000).ADS CAS Article PubMed PubMed Central Google Scholar 2.Hamilton, H., Caballero, S., Collins, A. G. & Brownell, R. L. Evolution of river dolphins. Proc. R. Soc. Lond. B 268, 549–556. https://doi.org/10.1098/rspb.2000.1385 (2001).CAS Article Google Scholar 3.Geisler, J. H., McGowen, M. R., Yang, G. & Gatesy, J. A supermatrix analysis of genomic, morphological, and paleontological data from crown Cetacea. BMC Evol. Biol. 11(1), 1–33 (2011).Article Google Scholar 4.Reeves, R. R., Smith, B. D. & Kasuya, T. Biology and conservation of freshwater cetaceans in Asia. In IUCN, Gland and Cambridge. IUCN SSC Occasional Paper, Vol. 23 (2000).5.Turvey, S. T. et al. First human caused extinction of a cetacean species?. Biol. Lett. 3, 537–540. https://doi.org/10.1098/RSBL.2007.0292 (2007).Article PubMed PubMed Central Google Scholar 6.da Silva, V. M. F. Aspects of the biology of the Amazonian dolphins genus Inia and Sotalia fluviatilis (The University of Cambridge, 1994). Google Scholar 7.Hrbek, T., da Silva, V. M. F., Dutra, N., Gravena, W. & Martin, A. R. A new species of river dolphin from Brazil or: How little do we know our biodiversity?. PLoS ONE 9, e83623. https://doi.org/10.1371/journal.pone.0083623 (2014).ADS CAS Article PubMed PubMed Central Google Scholar 8.Mosquera-Guerra, F. Mercury in populations of river dolphins of the Amazon and Orinoco basins. EcoHealth 16(4), 743–758. https://doi.org/10.1007/s10393-019-01451-1 (2019).CAS Article PubMed Google Scholar 9.Martin, A. R. & da Silva, V. M. F. Number, seasonal movements and residency characteristics of river dolphins using an Amazonian floodplain lake system. Can. J. Zool. 82, 1307–1315. https://doi.org/10.1139/z04-109 (2004).Article Google Scholar 10.Martin, A. R. & da Silva, V. M. F. River dolphins and flooded forest: Seasonal habitat use and sexual segregation of botos Inia geoffrensis in an extreme cetacean environment. J. Zool. 263, 295–305. https://doi.org/10.1017/S095283690400528X (2004).Article Google Scholar 11.da Silva, V. M. F. Inia geoffrensis. The IUCN Red List of Threatened Species. https://doi.org/10.2305/IUCN.UK.2018-2.RLTS.T10831A50358152.en (2018).12.ICMBio/MMA. Livro Vermelho da Fauna Brasileira Ameaçada de Extinção. (I). (2018).13.Brum, S. M. et al. Conservation of Amazonian aquatic mammals. Aquat. Conserv. Mar. Freshw. Ecosyst. 31(1068–1086), 2021. https://doi.org/10.1002/aqc.3590 (2021).Article Google Scholar 14.Trujillo, F., Crespo, E., Van Damme, P. A. & Usma J. S. The Action Plan for South American River Dolphins 2010–2020 (WWF, Fundación Omacha, WDS, WDCS, Solamac, 2010).15.Araújo, C. C. & Wang, J. Y. The dammed river dolphins of Brazil: Impacts and conservation. Oryx 49(1), 17–24. https://doi.org/10.1017/S0030605314000362 (2015).Article Google Scholar 16.Barbosa, M. S. et al. Total mercury and methylmercury in river dolphins (Cetacea: Iniidae: Inia spp.) in the Madeira River Basin, Western Amazon. Environ. Sci. Pollut. Res. https://doi.org/10.1007/s11356-021-13953-z (2021).Article Google Scholar 17.Alves, L. C. P. S., Zappes, C. A. & Andriolo, A. Conflicts between river dolphins (Cetacea: Odontoceti) and fisheries in the Central Amazon: A path toward tragedy?. Zoologia 29(5), 420–429. https://doi.org/10.1590/S1984-46702012000500005 (2012).Article Google Scholar 18.da Silva, V. M. F. & Martin, R. Status, threats, conservation initiatives and possible solutions for Inia geoffrensis and Sotalia fluviatilis in Brazil. In The Action Plan for South American River Dolphins 2010–2010 (eds Trujillo, F. et al.) (WWF – Fundación Omacha – WCS – WDCS – Solamac, 2010). Google Scholar 19.do Amaral, K. B. et al. Reassessment of the franciscana Pontoporia blainvillei (Gervais & d’Orbigny, 1844) distribution and niche characteristics in Brazil. J. Exp. Mar. Biol. Ecol. 508, 1–12. https://doi.org/10.1016/j.jembe.2018.07.010 (2018).Article Google Scholar 20.Zerbini, A. N., Secchi, E., Crespo, E., Danilewicz, D. & Reeves, R. Pontoporia blainvillei (errata version published in 2018). The IUCN Red List of Threatened Specieshttps://doi.org/10.2305/IUCN.UK.2017-3.RLTS.T17978A50371075.en.21.Paso Viola, M. N. et al. Diet composition of franciscana dolphin Pontoporia blainvillei from southern Buenos Aires, Argentina and its interaction with fisheries. Rev. Biol. Mar. Oceanogr. 49, 393–400. https://doi.org/10.4067/S0718-19572014000200019 (2014).Article Google Scholar 22.Romero, M. B., Polizzi, P., Chiodi, L., Das, K. & Gerpe, M. The role of metallothioneins, selenium and transfer to offspring in mercury detoxification in Franciscana dolphins (Pontoporia blainvillei). Mar. Pollut. Bull. 109(1), 650–654. https://doi.org/10.1016/j.marpolbul.2016.05.012 (2016).CAS Article PubMed Google Scholar 23.Barbosa, A. P. M. et al. Transplacental transfer of persistent organic pollutants in La Plata dolphins (Pontoporia blainvillei; Cetartiodactyla, Pontoporiidae). Sci. Total Environ. 631, 239–245. https://doi.org/10.1016/j.scitotenv.2018.02.325 (2018).ADS CAS Article PubMed Google Scholar 24.Denuncio, P. et al. Plastic ingestion in Franciscana dolphins, Pontoporia blainvillei (Gervais and d’Orbigny, 1844), from Argentina. Mar. Pollut. Bull. 62(8), 1836–1841. https://doi.org/10.1016/j.marpolbul.2011.05.003 (2011).CAS Article PubMed Google Scholar 25.Smith, K. F., Acevedo-Whitehouse, K. & Pedersen, A. B. The role of infectious diseases in biological conservation. Anim. Conserv. 12, 1–12. https://doi.org/10.1111/j.1469-1795.2008.00228.x (2009).Article Google Scholar 26.Woodard, J. C., Zam, S. G., Caldwell, D. K. & Caldwell, M. C. Some parasitic diseases of dolphins. Pathol. Vet. 6, 257–272 (1969).CAS PubMed Google Scholar 27.Ostenrath, F. Some remarks on therapy of mycotic and bacteriological skin diseases in freshwater dolphins, Inia geoffrensis. Aquat. Mamm. 4, 49–51 (1976). Google Scholar 28.Pier, G. B. & Madin, S. H. Streptococcus iniae sp. nov., a beta-hemolytic Streptococcus isolated from an amazon freshwater dolphin, Inia geoffrensis. Int. J. Syst. Evol. Microbiol. 26, 545–553. https://doi.org/10.1099/00207713-26-4-545 (1976).Article Google Scholar 29.Bonar, C. J. & Wagner, R. A. A third report of ‘golf ball disease’ in an Amazon River dolphin (Inia geoffrensis) associated with Streptococcus iniae. J. Zoo Wildl. Med. 34, 296–301. https://doi.org/10.1638/1042-7260(2003)034[0296:ATROGB]2.0.CO;2 (2003).Article PubMed Google Scholar 30.Bonar, C. J. et al. A retrospective study of pathologic findings in the Amazon and Orinoco river dolphin (Inia geoffrensis) in captivity. J. Zoo Wildl. Med. 38(2), 177–219. https://doi.org/10.1638/1042-7260(2007)038[0177:ARSOPF]2.0.CO;2 (2007).Article PubMed Google Scholar 31.Rodrigues, T. C. S., Díaz-Delgado, J., Catão-Dias, J. L., da Luz Carvalho, J. & Marmontel, M. Retrospective pathological survey of pulmonary disease in free-ranging Amazon river dolphin Inia geoffrensis and tucuxi Sotalia fluviatilis. Dis. Aquat. Org. 131(1), 1–11. https://doi.org/10.3354/dao03280 (2018).Article Google Scholar 32.Sacristán, C. et al. Novel herpesviruses in riverine and marine cetaceans from South America. Acta Trop. 190, 220–227. https://doi.org/10.1016/j.actatropica.2018.11.021 (2019).Article PubMed Google Scholar 33.Ruoppolo, V., Vanstreels, R. E., Marigo, J. & Catão-Dias, J. L. Unusual incidence of chronic pneumonia associated with cholesterol deposits in stranded and bycaught franciscanas Pontoporia blainvillei. Dis. Aquat. Org. 92, 75–81. https://doi.org/10.3354/dao02263 (2010).Article Google Scholar 34.Sánchez-Sarmiento, A. M. et al. Molecular, serological, pathological, immunohistochemical and microbiological investigation of Brucella spp. in marine mammals of Brazil reveals new cetacean hosts. Transboundary Emerg. Dis. 66(4), 1674–1692. https://doi.org/10.1111/tbed.13203 (2019).CAS Article Google Scholar 35.Fuentes-Castillo, D. et al. Colistin-resistant Enterobacter kobei carrying mcr-9.1 and blaCTX-M-15 infecting a critically endangered franciscana dolphin (Pontoporia blainvillei), Brazil. Transboundary Emerg. Dis. 00, 1–7. https://doi.org/10.1111/tbed.13980 (2021).Article Google Scholar 36.Marigo, J. et al. Parasites of Pontoporia blainvillei from São Paulo and Paraná States, Brazil. LAJAM 1(1), 115. https://doi.org/10.5597/lajam00015 (2002).Article Google Scholar 37.Luque, J. L. et al. Checklist of helminth parasites of cetaceans from Brazil. Zootaxa 2548(1), 57–68. https://doi.org/10.11646/zootaxa.2548.14 (2010).MathSciNet Article Google Scholar 38.Marigo, J. et al. Genetic diversity and population structure of Synthesium pontoporiae (Digenea, Brachycladiidae) linked to its definitive host stocks, the endangered Franciscana dolphin, Pontoporia blainvillei (Pontoporiidae) off the coast of Brazil and Argentina. J. Helminthol. 89(1), 19–27. https://doi.org/10.1017/S0022149X13000540 (2015).CAS Article PubMed Google Scholar 39.ICTV (International Committee on Taxonomy of Viruses). Virus Taxonomy: 2019 Release. EC 51, Berlin, Germany, July 2019. https://talk.ictvonline.org/ictv-reports/ictv_9th_report/dsdna-viruses-2011/w/dsdna_viruses/89/herpesvirales (2020).40.Esperón, F., Fernández, A. & Sánchez-Vizcaíno, J. M. Herpes simplex-like in a bottlenose dolphin stranded in Canary Islands. Dis. Aquat. Org. 81, 73–76. https://doi.org/10.3354/dao01915 (2008).Article Google Scholar 41.Maness, H. T. et al. Phylogenetic analysis of marine mammal herpesviruses. Vet. Microbiol. 149, 23–29. https://doi.org/10.1016/j.vetmic.2010.09.035 (2011).ADS Article PubMed Google Scholar 42.Smolarek-Benson, K. A. et al. Identification of novel alpha- and gammaherpesviruses from cutaneous and mucosal lesions of dolphins and whales. J. Virol. Methods 136, 261–266. https://doi.org/10.1016/j.jviromet.2006.03.033 (2006).CAS Article PubMed Google Scholar 43.Arbelo, M. et al. Herpesvirus infection with severe lymphoid necrosis affecting a beaked whale stranded in the Canary Islands. Dis. Aquat. Org. 89, 261–264. https://doi.org/10.3354/dao02208 (2010).Article Google Scholar 44.Burek-Huntington, K. A., Dushane, J. L., Goertz, C. E., Romero, C. H. & Raverty, S. A. Morbidity and mortality in stranded Cook Inlet beluga whales Delphinapterus leucas. Dis. Aquat. Org. 114, 45–60. https://doi.org/10.3354/dao02839 (2015).Article Google Scholar 45.Van Elk, C. et al. Central nervous system disease and genital disease in harbor porpoises (Phocoena phocoena) are associated with different herpesviruses. Vet. Res. 47, 28. https://doi.org/10.1186/s13567-016-0310-8 (2016).CAS Article PubMed PubMed Central Google Scholar 46.Bellière, E. N. et al. Presence of herpesvirus in striped dolphins stranded during the Cetacean morbillivirus epizootic along the Mediterranean Spanish coast in 2007. Adv. Virol. 155, 1307–1311. https://doi.org/10.1007/s00705-010-0697-x (2010).CAS Article Google Scholar 47.Burdett-Hart, L. B. et al. Skin lesions on common bottlenose dolphins (Tursiops truncatus) from three sites in the Northwest Atlantic, USA. PLoS ONE 7, e33081. https://doi.org/10.1371/journal.pone.0033081 (2012).ADS CAS Article Google Scholar 48.Van Beurden, S. J. et al. Identification of a novel gammaherpesvirus associated with (muco)cutaneous lesions in harbour porpoises (Phocoena phocoena). Adv. Virol. 160, 3115–3120. https://doi.org/10.1007/s00705-015-2607-8 (2015).CAS Article Google Scholar 49.Van Elk, C. E., Van de Bildt, M. W., De Jong, A. A., Osterhaus, A. D. & Kuiken, T. Herpesvirus in bottlenose dolphins (Tursiops truncatus): Cultivation, epidemiology, and associated pathology. J. Wildl. Dis. 45, 895–906. https://doi.org/10.7589/0090-3558-45.4.895 (2009).Article PubMed Google Scholar 50.Sierra, E. et al. Herpesvirus-associated genital lesions in a stranded striped dolphin (Stenella coeruleoalba) in the Canary Islands, Spain. J. Wildl. Dis. 51, 696–702. https://doi.org/10.7589/2014-07-185 (2015).CAS Article PubMed Google Scholar 51.Ewing, R. Y. et al. Macroscopic and histopathologic findings from a mass stranding of rough-toothed dolphins (Steno bredanensis) in 2005 on Marathon Key, Florida, USA. Front. Vet. Sci. 7, 572. https://doi.org/10.3389/fvets.2020.00572 (2020).Article PubMed PubMed Central Google Scholar 52.Lecis, R. et al. First gammaherpesvirus detection in a free-living Mediterranean bottlenose dolphin. J. Zoo Wildl. Med. 45, 922–925. https://doi.org/10.1638/2014-0019.1 (2014).Article PubMed Google Scholar 53.Seade, G. et al. Herpesviral infection in a Guiana dolphin (Sotalia guianensis) from the northern coast of Brazil. J. Vet. Diagn. Investig. 29(6), 877–879. https://doi.org/10.1177/1040638717727794 (2017).Article Google Scholar 54.Sierra, E. et al. Retrospective study of etiologic agents associated with nonsuppurative meningoencephalitis in stranded cetaceans in the Canary Islands. J. Clin. Microbiol. 52(7), 2390–2397. https://doi.org/10.1128/JCM.02906-13 (2014).Article PubMed PubMed Central Google Scholar 55.Vargas-Castro, I. et al. Alpha- and gammaherpesviruses in stranded striped dolphins (Stenella coeruleoalba) from Spain: First molecular detection of gammaherpesvirus infection in central nervous system of odontocetes. BMC Vet. Res. 16(1), 288. https://doi.org/10.1186/s12917-020-02511-3 (2020).CAS Article PubMed PubMed Central Google Scholar 56.Bento, M. C. et al. Herpesvirus infection in marine mammals: A retrospective molecular survey of stranded cetaceans in the Portuguese coastline. Infect. Genet. Evol. 67, 222–233. https://doi.org/10.1016/j.meegid.2018.11.013 (2019).CAS Article PubMed Google Scholar 57.das Neves, C. G., Sacristán, C., Madslien, K., Li, H. & Tryland, M. Gammaherpesvirus in cervid species from Norway: Characterization of a new virus in wild and semi-domesticated reindeer. Viruses 12(8), 876. https://doi.org/10.3390/v12080876 (2020).CAS Article PubMed Central Google Scholar 58.Nicolas de Francisco, O. et al. Neurological disease, neoplasms and novel gammaherpesviruses in critically endangered captive European minks (Mustela lutreola). Transboundary Emerg. Dis. 68, 552–564. https://doi.org/10.1111/tbed.13713 (2020).CAS Article Google Scho
https://www.nature.com/articles/s41598-021-04059-0
Highly divergent herpesviruses in threatened river dolphins from Brazil