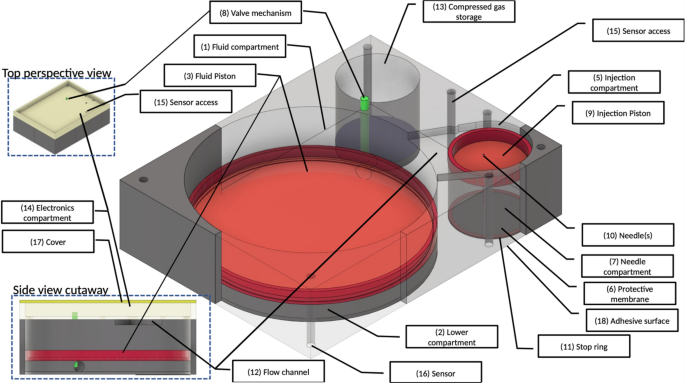
Hypoxia driven opioid targeted automated device for overdose rescue Opioid use disorder has been designated a worsening epidemic with over 100,000 deaths due to opioid overdoses recorded in 2021 alone. Unintentional deaths due to opioid overdoses have continued to rise inexorably. While opioid overdose antidotes such as naloxone, and nalmefene are available, these must be administered within a critical time window to be effective. Unfortunately, opioid-overdoses may occur in the absence of antidote, or may be unwitnessed, and the rapid onset of cognitive impairment and unconsciousness, which frequently accompany an overdose may render self-administration of an antidote impossible. Thus, many lives are lost because: (1) an opioid overdose is not anticipated (i.e., monitored/detected), and (2) antidote is either not present, and/or not administered within the critical frame of effectiveness. Currently lacking is a non-invasive means of automatically detecting, reporting, and treating such overdoses. To address this problem, we have designed a wearable, on-demand system that comprises a safe, compact, non-invasive device which can monitor, and effectively deliver an antidote without human intervention, and report the opioid overdose event. A novel feature of our device is a needle-stow chamber that stores needles in a sterile state and inserts needles into tissue only when drug delivery is needed. The system uses a microcontroller which continuously monitors respiratory status as assessed by reflex pulse oximetry. When the oximeter detects the wearer’s percentage of hemoglobin saturated with oxygen to be less than or equal to 90%, which is an indication of impending respiratory failure in otherwise healthy individuals, the microcontroller initiates a sequence of events that simultaneously results in the subcutaneous administration of opioid antidote, nalmefene, and transmission of a GPS-trackable 911 alert. The device is compact (4 × 3 × 3 cm), adhesively attaches to the skin, and can be conveniently worn on the arm. Furthermore, this device permits a centralized remotely accessible system for effective institutional, large-scale intervention. Most importantly, this device has the potential for saving lives that are currently being lost to an alarmingly increasing epidemic. Opioid use disorder has been designated as a worsening epidemic by the Center for Disease Control and Prevention (CDC). Approximately 100,000 people died from opioid-involved overdoses in 2021 according to the National Center for Health Statistics1,2, and the economic cost associated with such overdoses in the United States totaled $550 billion in 20173. The increasing societal burden of the opioid use disorder and accompanying opioid-involved overdoses has raised the question of how deaths due to opioid overdoses can be better prevented.While FDA-approved antidotes for opioid overdoses are available, such as naloxone4, and nalmefene5 they are effective in saving lives only if administered within a critically short time-frame after the overdose has occurred. Unfortunately, opioid-overdoses may occur in the absence of antidote6, or may be unwitnessed, and the rapid onset of cognitive impairment and unconsciousness, which frequently accompany an overdose may render self-administration of an antidote impossible7. Thus, many lives are lost because: (1) an opioid overdose is not anticipated (i.e., monitored/detected), and (2) antidote is either not present, and/or not administered within the critical window of effectiveness. The aim of this investigation is to design a safe, compact, non-invasive device that can monitor, and report an opioid overdose, and effectively deliver an antidote without human intervention.Intravenous, and intraarterial, cannula-based drug systems have been widely used for therapeutic drug delivery for extended periods of time in a variety of medical illnesses. Such systems require frequent monitoring and replacement of parts because of the possibility of infection, among other untoward effects8. Other devices, such as insulin pumps, although somewhat less invasive since they are subcutaneous, also require frequent replacement of both delivery device and medication and have the potential adverse effects of scarring and bleeding9. More recently, a subcutaneous implantable device has been proposed for treatment of opioid overdoses10. The latter utilizes a burst release design, which has the advantage of on-demand administration of antidote. The device, however, has the disadvantage of housing the medication reservoir subcutaneously, and therefore has an element of invasiveness associated with both implantation and use, including the potential untoward effects of infection, tissue injury, and leakage of drug systemically. It is also quite cumbersome to wear10. To overcome these deficiencies, we have designed a wearable automated, burst release, on-demand, compact device for monitoring, reporting, and treating opioid overdoses. Our device uses a simple, integrated power supply to drive a sensor that continuously monitors blood oxygenation via oximetry, and a microcontroller, which responds to critically low oxygen levels, consistent with an overdose, by orchestrating the injection of an opioid antagonist, nalmefene subcutaneously. The microcontroller will also transmit a GPS-trackable 911 alert to inform of the overdose and can be interrogated remotely to provide relevant biometric data. The delivery device is designed to be compact (4 × 3 × 3 cm), and to conveniently attach adhesively to the skin of the upper arm. 1. Control and monitoring: A microcontroller-based design is used for monitoring and control using a set of sensors. Communication and tracking will be provided by Blue-tooth, WIFI, cellular and GPS tracking modules. A rechargeable battery will provide power to the system. 2. Mechanical Design: Needle insertion and drug delivery system. The overall mechanical design of the injection device (patent-pending 63/061666) and delivery system (patent pending 63/081579) is presented in Fig. 1. The Main Fluid Compartment [Fig. 1] is divided into two parts by the Fluid piston [Fig. 1, (3)]. The Upper compartment [Fig. 1, (1)] contains fluid (i.e., opioid antidote) and the Lower compartment [(Fig. 1, (2)] contains air. The Lower compartment (Fig. 1, (2)] is connected via a Control valve [Fig. 1, (8)] to the Compressed gas compartment [Fig. 1, (13)]. The Upper main fluid compartment [Fig. 1, (1)] is connected to the Injection compartment [Fig. 1, (5)] via a Flow channel [Fig. 1, side view cutaway, (12)]. When triggered, the Valve [Fig. 1, (8)] is opened, and gas enters the Lower fluid compartment [Fig. 1, (2)] pushing the Fluid piston [Fig. 1, (3)] upwards. This drives fluid (i.e., opioid antidote) into the Injection compartment [Fig. 1, (5)], which then pushes the Injection piston [Fig. 1, (9)] downward. Under the force exerted by the downward movement of the Injection piston [Fig. 1, (9)], the Hypodermic Needle [Fig. 1, (10)] passes through the Protective membrane [Fig. 1, (6)] and penetrates the tissue. Fluid flows only when the Needle [Fig. 1, (10)] is fully inserted into the wearer’s subcutaneous tissue. The Stop ring [Fig. 1, (11)] then inhibits further movement of the Injection piston [Fig. 1, (9)]. The relative movement of pistons and inner assembly is governed by a valve controller. Piston position sensors provide feedback control by which the fluid injection rate and profile are controlled. An algorithm controls the Gas Valve [Fig. 1, (8)] to provide a pre-programed drug flow rate. The system is designed to deliver an initial 1 milliliter dose of antidote followed by two additional doses, at 5-minute intervals, in the event of continued oxygen desaturation at either time interval. 3. Simulation and device parameters: The mechanical simulation, and device key parameters are given in Table 1. Device maximum dimensions are 4 cm (length), 3 cm (width), 3 cm (height), calculated as follows: (1) Length: Fluid and injection compartments require 3.14 cm. An additional 0.86 cm has been added for walls, resulting in a final length of approximately 4 cm. Width: 2.14 cm width accommodates the fluid compartment (and thus both injection and gas compartments). An additional 0.86 cm has been added for wall thickness, thus generating a final width of 3 cm. Height: 1.1 cm accommodates the cylinder height. An additional 1.9 cm has been added for wall thickness, and the electrical components, resulting in a final height of 3 cm. Compressed gas weight is less than 15 g. 4. Needle: The hypodermic needle to be used to deliver antidote subcutaneously will be 30 gauge, and 8 mm in length (JPN Nanoneedle11,). 5. Nalmefene: Nalmefene hydrochloride (cGMP grade) will be purchased from Rusan Pharma Ltd. (Mumbai, India) and prepared as described by others12. The sterile, pyrogen-free preparation for subcutaneous administration will be identical to that listed for Revex (nalmefene hydrochloride)13 (https:// www.accessdata.fda.gov/drugsatfda_docs/label/2006/020459s006lbl.pdf). The concentration of drug for administration will be 1 mg per milliliter. 6. Oximeter: A reflectance pulse oximeter is integrated into the microcontroller with sensors located as shown in Fig. 1 [item 16]. The microcontroller is programmed to engage the injection device when the percent saturation of hemoglobin with oxygen is less than, or equal to 90%. The biceps location for sensing oxygen saturation has been validated in a recent report14. 7. Selection Criteria for Wearing the Device: Our patient population will consist of opioid addicts, particularly those who have recently undergone withdrawal therapy and have elected not to enter a medically assisted arm of treatment, since this group is at high risk for recidivism. Patients entering the protocol will undergo a clinical examination consisting of a history, physical exam and baseline oximetry evaluation. Patients will be excluded from wearing the device if (a) there is a prior, or current history of cardiac or pulmonary disease that might predispose to oxygen desaturation, (b) the physical exam reveals evidence of heart or lung disease that might also predispose to oxygen desaturation, and/or (c) the individual’s percent saturation of hemoglobin with oxygen (SpO2) at rest is less than 93%. 8. Patient follow-up: Individuals who wish to wear the device must submit initially to weekly clinic visits, at which time the device will be checked, components within the device will be replaced if necessary, and the device will be interrogated for compliance. Non-compliance will result in removal from the protocol. Figure 1Design overview of the wearable automated self-activated opioid-overdose antidote injection system. Insets show top and side cutaway views. See text for further details. Table 1 Simulation and device parameters. This device is designed to be compact and wearable and attaches to the skin using an adhesive surface (Fig. 1, 18). A novel feature of our device is a needle-stow chamber that stores needles in a sterile state and inserts needles into tissue only when drug delivery is needed (see Fig. 1 for details). Figure 2 shows the overall function of the system. An algorithm continuously monitors physiological state of the wearer for an opioid-overdose. A mechanical pump system is activated upon detection of an overdose which inserts the hypodermic needles into tissue, injects the antidote, and simultaneously transmits alerts and data to designated remote systems (e.g., 911 and remote servers). Furthermore, this device can be interrogated remotely for a system health check and the state of the wearer.Figure 2Schematic overview of the opioid-overdose delivery system.The device is composed of two main systems: (1) a microcontroller-based monitoring, reporting, and control system, and (2) a drug delivery system that stores antidote, and stowed hypodermic needle, which, when triggered, inserts the needle into the subcutaneous tissue of the wearer and delivers the antidote (Fig. 1).A reflectance pulse oximeter15 serves as a sensor which continuously feeds physiological data to the microcontroller in the form of percent saturation of hemoglobin with oxygen (SpO2). The microcontroller is programmed to be activated at a SpO2 of less than, or equal to 90%, which corresponds to an approximate partial pressure of oxygen in arterial blood (PaO2) of 60 mm of mercury (normal 80–100 mm of mercury;16), and indicates a failure of oxygenation consistent with an opioid overdose in the appropriate setting7. Once an opioid overdose is detected, the microcontroller simultaneously transmits a GPS-trackable, 911 alert, and engages the drug delivery system, which then immediately inserts a needle into the subcutaneous tissue of the wearer and delivers 1 ml of antidote. Needle insertion and drug delivery will be complete within ~ 1.2 s of overdose detection (see Fig. 3). The microcontroller is programmed to re-engage the injection device at 5 min intervals × 2 in the event of continued oxygen desaturation at either time interval.Figure 3Dynamic model simulation of the mechanical pump system shown for the initial injection cycle. Left panels show time evolution of dynamic variables: (A) position of main piston (red trace) and needle piston (blue trace), (B) flow of drug through the needle, (C) volume of drug injected into the subcutaneous tissue, (D) force applied on the needles. Right panels: State of the device at various time points. Balloons 1–4 mark time points which correlate with dynamic states of the device as shown in the left panels 1–4. Shown is a replica of the device mechanics for the initial cycle of engagement following triggering when an SpO2 of less than, or equal to 90% has been sensed by the oximeter. See text for further details.Device administration and useThe mechanical design of the device has been described in detail above. Briefly, based on a trigger, gas enters the lower part of the Fluid compartment [Fig. 1, (2)], which creates pressure, pushing antidote, stored in the upper Fluid compartment [Fig. 1 (1)], into the Injection compartment [Fig. 1, (5)]. This then drives the Injection piston [Fig. 1, (9)] downward thereby inserting the Hypodermic needle [Fig. 1, (10)] into the subcutaneous tissue. Once needle placement has been completed, antidote is infused into the tissue. Further movement of the inner assembly is prevented by a Stop ring (Fig. 1, 11), and the initial cycle of drug administration ends. Sensors [Fig. 1, (16)] embedded in the bottom of the device provide for controlling valve opening, and/or other biometric data to be used by smart control algorithms running on the microcontroller, thus governing the rate of drug injection. The device is equipped with a sufficient supply of gas, and antidote reservoir to accommodate a total of three infusions.Mathematica
https://www.nature.com/articles/s41598-021-04094-x
Hypoxia driven opioid targeted automated device for overdose rescue