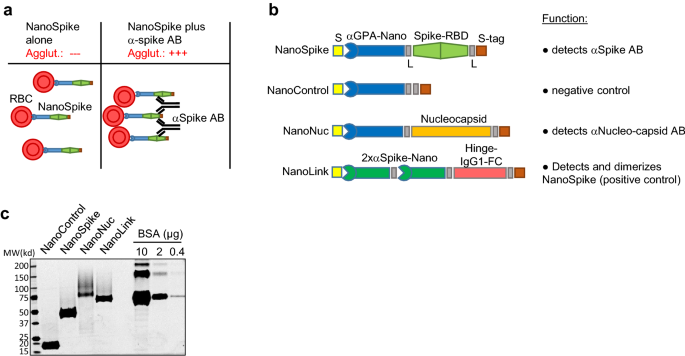
A rapid and affordable point of care test for antibodies against SARS-CoV-2 based on hemagglutination and artificial intelligence interpretation Vanessa Redecke1, Kazuki Tawaratsumida1, Erin T. Larragoite2, Elizabeth S. C. P. Williams2, Vicente Planelles2, Adam M. Spivak3, Lincoln Hirayama4, Marc Elgort4, Shane Swenson5, Rick Smith5, Bryan Worthen5, Russ Zimmerman5, Patricia Slev4, Ben Cahoon5, Mark Astill4 & Hans Häcker1 Scientific Reports 11, Article number: 24507 (2021) Cite this article DiseasesHealth careMicrobiology Diagnostic tests that detect antibodies (AB) against SARS-CoV-2 for evaluation of seroprevalence and guidance of health care measures are important tools for managing the COVID-19 pandemic. Current tests have certain limitations with regard to turnaround time, costs and availability, particularly in point-of-care (POC) settings. We established a hemagglutination-based AB test that is based on bi-specific proteins which contain a dromedary-derived antibody (nanobody) binding red blood cells (RBD) and a SARS-CoV-2-derived antigen, such as the receptor-binding domain of the Spike protein (Spike-RBD). While the nanobody mediates swift binding to RBC, the antigen moiety directs instantaneous, visually apparent hemagglutination in the presence of SARS-CoV-2-specific AB generated in COVID-19 patients or vaccinated individuals. Method comparison studies with assays cleared by emergency use authorization demonstrate high specificity and sensitivity. To further increase objectivity of test interpretation, we developed an image analysis tool based on digital image acquisition (via a cell phone) and a machine learning algorithm based on defined sample-training and -validation datasets. Preliminary data, including a small clinical study, provides proof of principle for test performance in a POC setting. Together, the data support the interpretation that this AB test format, which we refer to as ‘NanoSpot.ai’, is suitable for POC testing, can be manufactured at very low costs and, based on its generic mode of action, can likely be adapted to a variety of other pathogens. The COVID-19 pandemic caused by SARS-CoV-2 passed its first anniversary with more than 2.5 million deaths worldwide. While initial countermeasures were largely restricted to therapeutic interventions for those with severe infections and prophylactic social distancing, recent clinical studies demonstrated efficacy for various SARS-CoV-2 vaccines, whose deployment is underway in many countries. Still, proper pandemic management hinges on the availability of diagnostic tests, including those revealing the presence of antibodies (AB) against the virus. On a population scale, results obtained by these tests reveal seroprevalence and the dynamics of virus spread, helping to predict susceptibility of a population and guiding health care decisions. On an individual level, such test results indicate previous SARS-CoV-2 infection or successful vaccination and have likely high predictive value for disease susceptibility. While numerous AB tests are available, they do have certain limitations. Tests performed in reference laboratories, such as enzyme-linked immunosorbent assays (ELISA) and related technologies, require special equipment, are relatively expensive and typically associated with long turn-around times from sample acquisition to results. Point-of-care (POC) tests, like the lateral flow immuno-assay (LFIA or LFA), can be conducted with minimal laboratory equipment, but typically show reduced sensitivity compared to tests conducted in reference labs and are also relatively expensive1,2,3. Moreover, analysis is performed by visual inspection, which is subjective in nature and thus affects specificity and sensitivity dependent on more or less conservative interpretation4. An alternative format for detection of antigen-specific antibodies in whole blood are hemagglutination tests (HAT). This format is typically based on bi-specific proteins with one moiety, e.g. an AB, binding to red blood cells (RBC) and the other moiety encompassing the antigen, e.g. a virus protein as target for the investigated AB. Antigen-specific AB contained in whole blood bind to the test antigen and mediate visible agglutination of RBC within seconds to minutes (Fig. 1a)5. Tests based on such fusion proteins were validated in comparison to a number of FDA-approved assays including ELISA, Western blotting and immunofluorescence assays (IFA), demonstrating remarkably comparable performance5,6. A more recent development of this principle employed as RBC-binding moiety a dromedary-derived single variable domain on a heavy chain (VHH)(nanobody) against Glycophorin A (GPA), which is expressed at high levels on RBC7. In a proof of principle assay, an E. coli-expressed fusion protein of this nanobody (IH4) and HIV p24 demonstrated high affinity binding of GPA (KD = 33.72 nM) and the detection of p24-directed AB in the plasma of an HIV-positive patient7. HAT for AB against SARS-CoV2 have been developed, however, the utility of these tests in POC settings is limited and visual readout remains subjective (see ‘Discussion’ section)8,9,10. Here, we describe the development of a test, which is based on a modified form of the nanobody targeting GPA and the Spike-RBD as antigen. Analyses related to protein production, RBC-binding and hemagglutination in the presence of SARS-CoV-2-specific antibodies suggest excellent test performance. Moreover, artificial intelligence (AI)-based analysis of digital images obtained via cell phone suggest that test interpretation can be objectified and quantified by computer analysis. Given the simplicity, robustness and low production costs, this test may facilitate the assessment of AB responses against SARS-CoV-2 in POC settings, including those with limited resources, and thus contribute to pandemic management.Figure 1Recombinant proteins and test principle. (a) Principle of NanoSpike-mediated hemagglutination in the presence of αSpike AB. (b) Schematic presentation of recombinant proteins. S, secretion signal (IFNβ); αGPA-nano, GPA-specific nanobody; L, flexible linker; Spike, Spike-RBD of SARS-CoV-2; S-tag, Tandem-Strep-tag; 2xαSpike-Nano, tandem-fusion construct of two Spike-RBD-binding H11-D4 nanobodies, separated by flexible linker; Hinge-IgG1-FC, dimerizing Hinge-FC moiety of IgG1. (c) SyproRuby-stained SDS PAGE of 3 µg of indicated proteins that were expressed in Expi293F cells and purified by one-step affinity purification. Protein yield from 25 ml of Expi293F-cell cultures were 2 mg (NanoControl), 3.75 mg (NanoSpike), 0.55 mg (NanoNuc) and 1.25 mg (NanoLink). Defined amounts of BSA are shown for comparison.We chose the GPA-directed nanobody IH4 as starting point due to the known robustness of nanobodies and the availability of sequence information7. Two mutations were introduced to enable efficient protein secretion in mammalian cells (see ‘Methods’ section). As illustrated in Fig. 1b, we fused the IH4 nanobody initially to two viral antigens, i.e. the receptor-binding domain of the Spike protein (Spike-RBD) of SARS-CoV-2 (NanoSpike) and the Nucleocapsid (NanoNuc). The Spike-RBD was shown to represent a highly immunogenic region in various analyses11,12,13. Antibodies against the Spike-RBD were found to correlate well with neutralizing activity against the virus in both adults and children14,15,16. Moreover, Spike-RBD of SARS-CoV2 did not exhibit cross-reactivity with AB against SARS-CoV or seasonal CoV infections, providing the required predictive value to identify AB against SARS-CoV-217. Last, the majority of currently available vaccines are based on the Spike protein; as such, tests incorporating this protein will allow assessment of successful vaccination. AB against the Nucleocapsid of SARS-CoV2 are also found in the majority of SARS-CoV-2-infected individuals, and AB titers are particularly high in severe cases14. Since most vaccines are based on the Spike protein, Nucleocapsid-directed AB can potentially be used to distinguish if AB were obtained during natural infection or vaccination. However, as detailed below, experiments related to test sensitivity (and the potential cross-reactivity of full-length Nucleocapsid with AB against other Coronavirus strains) indicated superior properties of the Spike-RBD as test reagent. As such, we focused our studies primarily on Spike-RBD. In contrast to the original approach using E. coli as expression host, we focused on mammalian protein expression. Spike protein is physiologically targeted to the secretory pathway, which is likely required for proper protein folding and accompanied by glycosylation, which in turn may be relevant for the AB response and test specificity18. Thus, to allow for proper targeting to the secretory pathway, we added a secretion signal derived from Interferon-beta to the N-terminus of the fusion protein (Fig. 1b). The Nucleocapsid protein does not contain a secretion signal and is expressed primarily in the cytoplasm. However, initial expression studies suggested increased protein yield and purity when expressed as secreted protein. We therefore followed a similar strategy as used for the NanoSpike and added a secretion signal to the N-terminus (Fig. 1b). A tandem Strep-tag was fused to the C-terminus to allow for efficient one-step protein purification. Flexible glycine-serine-containing linkers were placed between the functional units of the fusion protein to avoid interference of the different domains. A similar protein lacking a viral antigen was cloned as negative control (NanoControl, Fig. 1b). Last, we designed a positive control protein. Here we took advantage of a recently published nanobody against the Spike-RBD, H11-D419. This nanobody was arranged in tandem to increase affinity and linked to the Hinge-FC-domain of IgG1, which forms constitutive dimers. Based on these characteristics, we would expect this protein (NanoLink) to act as surrogate for physiological AB directed against the Spike-RBD and, in the presence of NanoSpike, induce hemagglutination. We expressed these proteins first in small scale, then in larger scale experiments in Expi293F cells (Invitrogen), which are optimized for high-density growth in suspension. Protein expression and Strep-tag-based affinity purification were efficient, resulting in virtually homogenous protein preparations with robust yields (Fig. 1c, Supplementary Fig. 1).Functional activity of recombinant proteinsWe first confirmed binding of recombinant proteins to human RBC by flow cytometry (Fig. 2a). A kinetics analysis illustrates the rapid and quantitative process involved (Fig. 2b). We next tested if AB-mediated cross-linking of NanoSpike triggers hemagglutination. As shown in Fig. 3a, a αStrep-tag AB induced almost instant, clearly visible hemagglutination in the presence of NanoControl or NanoSpike. Neither protein showed any agglutinating activity on its own. Given that NanoControl binds RBC, but lacks a viral antigen, this protein controls efficiently for non-specific (antigen-independent) cross-linking activity. We note that the αStrep AB is a monoclonal IgG1 AB, suggesting that the valency of IgG is sufficient to trigger agglutination.Figure 2RBC-directed recombinant proteins bind RBC swiftly and quantitatively. (a) Flow cytometry analysis of human RBC that were left untreated (dark) or incubated with 30 µg/ml indicated proteins (light), followed by incubation with FITC-labeled AB against the Strep-tag. (b) Kinetics of RBC-binding of nanobody-fusion proteins analyzed by flow cytometry. αStrep-FITC AB alone (control) or together with indicated recombinant proteins was added to human RBC (time point 0) and binding was analyzed by flow cytometry during time.Figure 3Functional activity of recombinant proteins detecting SARS-CoV-2-specfic AB. (a) Hemagglutination assay of whole EDTA blood that was treated with NanoControl or NanoSpike protein (30 µg/ml) in the presence or absence of AB against the Strep-tag. (b) Hemagglutination assay of blood that was treated with NanoSpike (30 µg/ml) in the absence or presence of NanoLink at concentrations corresponding to a molar ratio of NanoSpike-to-NanoLink of 9:1, 3:1 and 1:1. Blood treated with NanoLink, NanoControl, NanoSpike or a combination of NanoSpike and αStrep AB is shown for comparison. Time points indicate the duration of agglutination. (c) Hemagglutination assay of blood from a convalescent COVID-19 patient that was treated with indicated concentrations of NanoSpike (c) or NanoNuc (d). Samples treated with NanoControl (30 µg/ml) or NanoSpike (30 µg/ml) plus NanoLink (14.3 µg/ml) serve as controls. Time points indicate the duration of agglutination. (d) Phase contrast microscopy of blood from a convalescent COVID-19-patient that was incubated with NanoControl and NanoSpike. (e) Phase contrast microscopy of blood from a convalescent COVID-19-patient and a COVID-19-negative control patient, which was incubated with NanoSpike.To simplify the interpretation of the agglutination test, we utilized the positive control protein (NanoLink) as depicted in Fig. 1b. Given its Spike-RBD-binding nanobody (H11-D4) and dimerizing IgG1 FC-domain, this protein is expected to mimic the function of physiological AB directed against the Spike protein and, as a consequence, to induce hemagglutination in the presence of NanoSpike. As shown in Fig. 3b, this is indeed the case. Up to a molar ratio of 9 to 1 of NanoSpike versus NanoLink, NanoLink was inducing clearly visible hemagglutination, while NanoLink alone remained without effect. As such, NanoLink and NanoControl recapitulate the appearance of a negative and positive test results, facilitating visual interpretation.Having established negative and positive controls, we used blood from a convalescent COVID-19 patient to test if NanoSpike and NanoNuc trigger hemagglutination in the presence of SARS-CoV-2-specific antibodies. For this purpose we titrated the recombinant proteins to establish robust conditions. In a range of 3–30 µg/ml, both, NanoSpike and NanoNuc, triggered hemagglutination, which became visible almost instantly and was fully developed between 1 and 3 min (Fig. 3c,d and Supplementary Fig. 2). Concentrations of recombinant protein above 30 µg/ml reduced agglutination in case of Nanospike, while concentrations below 3 µg/ml were less active in case of both, NanoSpike and NanoNuc. Proteins at 3 µg/ml were active, but induced slightly delayed agglutination in comparison to proteins at 10 and 30 µg/ml (Fig. 3c,d and Supplementary Fig. 2). Neither NanoSpike nor NanoNuc induced hemagglutination at any concentration in the blood of a healthy donor (Supplementary Fig. 3a,b).Based on these results, we continued our studies with proteins at 30 µg/ml. As expected, macroscopic hemagglutination was reflected by microscopically apparent formation of RBC-clusters induced by NanoSpike in the blood of a convalescent COVID-19-patient (Fig. 3d). NanoControl did not induce any visible cluster formation in the same blood. Likewise, NanoSpike failed to induce RBC-cluster formation in the blood of a COVID-19-negative control patient (Fig. 3e).Activity of the proteins was maintained for extended periods of time (up to 16 weeks) when stored in a refrigerator at 4 °C (Supplementary Fig. 4). Storage at room temperature (25 °C) and at 37 °C did also not show a loss in activity when assessed with undiluted serum; five-fold dilution of the serum indicated a slight reduction in activity, suggesting that storage at 4 °C (or subzero temperatures in a freezer) will be advantageous if the proteins are stored for extended period of times. We note that optimization of st
https://www.nature.com/articles/s41598-021-04298-1
A rapid and affordable point of care test for antibodies against SARS-CoV-2 based on hemagglutination and artificial intelligence interpretation