Assessment of SEAKER cell immunogenicity in an immunocompetent mouse model. (a) Experimental scheme to assess immunogenicity of β-Lac-MUC28z SEAKER cells in a syngeneic intraperitoneal ID8 tumor model. Sera were collected on Days 21, 24, 28, and 31 and tested for anti-β-Lac antibodies in panel b. In a separate experiment, ascites were recovered on Day 28 by peritoneal lavage and tested for the presence of SEAKER cells in panels c,d and β-Lac enzyme activity in panel e. (b) Detection anti-β-Lac antibodies in sera over 10 days following SEAKER cell engraftment (Days 21–31) (median with lines representing each individual mouse of n = 12; experiment was performed once). (c,d) Flow cytometry analysis of SEAKER cell (myc+) persistence among T cells (CD3+) and (e) nitrocefin cleavage-based quantitation of β-Lac enzyme activity in ascites recovered 7 days after SEAKER cell engraftment (Day 28) (representative data shown from n = 2 mice per group; on average, 25% of T cells were SEAKER-positive; experiment was performed once). SSC = side scatter. (f) In a third experiment, mice were treated as in panel a, but without cyclophosphamide pretreatment to maximize the antibody response, then sera were recovered 5 days after SEAKER cell engraftment (Day 26) and analyzed for anti-β-Lac antibodies (n = 1 mouse in untreated group; n = 5 mice in treated group; mean ± s.d. of n = 3 technical replicates from each mouse; experiment was performed once). Sera from the 4 mice showing anti-β-Lac antibodies were used for the ex vivo enzyme activity experiment in panel g. (g) Nitrocefin cleavage-based quantitation of enzyme activity of recombinant β-Lac treated with sera from untreated or SEAKER-treated mice from panel f (n = 2 mice in untreated group; mean ± s.d. of n = 4 mice in treated group; experiment was performed once).
https://www.nature.com/articles/s41589-021-00932-1
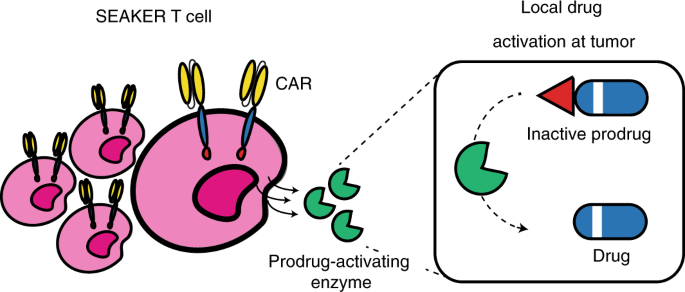
Engineering CAR-T cells to activate small-molecule drugs in situ