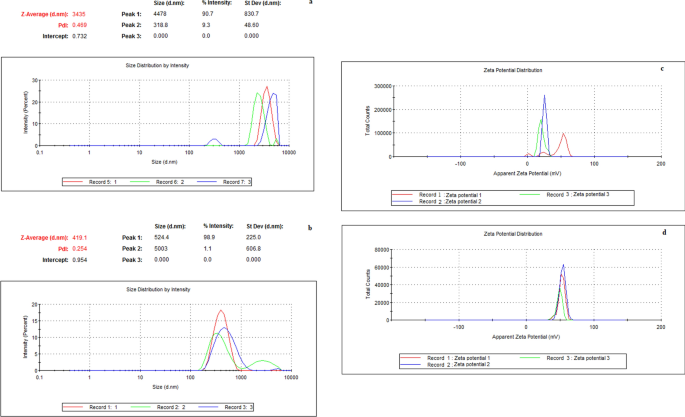
Antimicrobial and cytotoxic activity of electrosprayed chitosan nanoparticles against endodontic pathogens and Balb/c 3T3 fibroblast cells The aims of this study were to synthesize highly positively charged chitosan nanoparticles (Ch-Np) using the electrospraying technique, and to test their antimicrobial activity against endodontic pathogens, and cytotoxicity against fibroblast cells. Ch-Np were synthesized from low molecular weight chitosan (LMW-Ch) using the electrospraying technique, and characterized. The antimicrobial activity was evaluated against Streptococcus mutans, Enterococcus faecalis, and Candida albicans in their planktonic state using a Time-Kill Test performed by using broth micro-dilution technique, and against biofilm biomass using a microtiter plate biofilm assay. The cytotoxicity was evaluated using Balb/c 3T3 fibroblast cells with the standard MTT assay. Electrospraying of LMW-Ch produced Ch-Np with an average size of 200 nm, and a surface charge of 51.7 mV. Ch-Np completely eradicated S. mutans and E. faecalis in the planktonic state and showed fungistatic activity against C. albicans. Furthermore, it significantly reduced the biofilm biomass for all the tested microbial species [S. mutans (p = 0.006), E. faecalis (p < 0.0001), and C. albicans (p = 0.004)]. When tested for cytotoxicity using 3T3 cells, Ch-Np showed no cytotoxicity. In conclusion, the highly positively charged, colloidal dispersion of Ch-Np are effective as a biocompatible endodontic antimicrobial agent. The main cause of endodontic and periapical diseases is microbial contamination of the root canal system. This microbial contamination may range from bacterial, fungal to viral1,2. Thus, the aim of treating root canal infection is to eliminate microbial contamination from the entire root canal system3. Different treatment modalities are used to disinfect the root canal system, such as mechanical removal of the infected dentine4 in combination with antimicrobial agents in the form of root canal irrigants and intra-canal medicaments5. Other treatment modalities used to disinfect the root canal system include using laser therapy6, photodynamic therapy7, and ozone8.However, one of the main shortcomings of the current root canal treatment modalities is the inability to completely eradicate persistent pathogens. For instance, nickel titanium rotary instruments were found to result in variable unprepared surfaces and thus incomplete removal of all pathogens from the root canal system9,10,11. Furthermore, some microbial species such as Enterococcus faecalis were resistant to commonly used antimicrobial root canal irrigants and medicaments12. Similarly, photodynamic therapy resulted in only 80% reduction of resistant endodontic pathogens such as Actinomyces israelii, Fusobacterium nucleatum, Porphyromonas gingivalis, and Prevotella intermedia13. The use of different wavelengths in laser therapy to disinfect the root canal system also showed ineffective results14.Antimicrobial nanoparticles have shown a promising effect against resistant pathogens due to their unique physio-chemical properties15. The antimicrobial activity of nanoparticles against different microorganisms was found to be different from its original bulk state16. Polymeric nanoparticles have gained significant interest as new antimicrobial agents due to their biocompatibility and ability to eradicate microbial species by multiple mechanisms17. Chitosan is a natural polysaccharide that showed some antimicrobial activity at the macroscale level18. Furthermore, the presence of chitosan in a nano-scale form enhances its antimicrobial properties19. Chitosan nanoparticles (Ch-Np) can be synthesized using either cross-linking (chemical or physical) or drying techniques20. The drawback of using chemical cross-linking methods is the risk of synthesizing toxic and chemically less stable nanoparticles due to the effect of different types of solvents or cross-linkers used21. The limitation of using the physical cross-linking method to produce Ch-Np is the tendency of the Ch-Np to aggregate upon the addition of another polymer to produce the nanoparticles22.Electrospraying is an example of a drying technique that is used to synthesize various nanoparticles. It is a simple method based on the synthesis of solid nanoparticles from a liquid by an electrical force and hence the name electrospraying23. The mechanism of nanoparticle formation through electrospraying depends on the force that acts on the bulk liquid while passing through a needle at a constant rate under the influence of high electrical potential. The passage of the electrical current through a liquid droplet can create an electrical or 'Coulomb' force inside the droplet24. This electrical force will act against the cohesive force of the droplet caused by the surface tension of the dissolving liquid. Nanoparticles are formed when the Coulomb force exceeds the cohesive force, resulting in the droplets breaking into small particles on a nano-scale level, while the solvent evaporates from the droplet25.Thus, the aims of this study were firstly to synthesize highly positively charged Ch-Np using the electrospraying technique and determine their characteristics. The second aim was to test the antimicrobial activity of the synthesized Ch-Np against resistant endodontic pathogens in their planktonic state and against their biofilm biomass. The third aim was to evaluate the biocompatibility of the synthesized Ch-Np toward fibroblast cells.The null hypothesis of this study was that, electrospraying of LMW-Ch would not produce highly positively charged Ch-Np, that would not possess antimicrobial activity against endodontic pathogens in the planktonic state, nor against their biofilm biomass. Furthermore, these particles would possess a cytotoxic effect when tested using fibroblast cells.Low molecular weight chitosan (LMW-Ch) (Merck, Sigma Aldrich, Saint Louis, MO, USA) with 50–190 KDa and a 75–85% degree of deacetylation was used at a concentration of 6% by mass by dissolving it in 90% (v/v) Trifluoroacetic acid (TFA) ReagentPlus 99% (Merck, Sigma Aldrich, Saint Louis, MO, USA). The chitosan was allowed to disperse completely in TFA using a magnetic stirrer (Bibby Heated Magnetic Stirrer HB502, Sterilin, England) at 1000 rpm for 4 h at 50 °C.Synthesis of Ch-Np (electrospraying)The commercial LMW-Ch dispersion was dispensed in a syringe filled with a 19 mm gauge needle. The syringe was fixed in a 33 DDS dual drive independent channel syringe pump (Harvard apparatus, Massachusetts, MA, USA) for electrospraying. The chitosan colloidal suspension was electrosprayed in droplets at room temperature and a flow rate of 0.4 mL/h. A 25 kV potential was applied between the needle and the collector (aluminum foil) using a high voltage generator. TFA was evaporated from droplets during the time of flight and the dry chitosan nanoparticles were collected upon an aluminum foil collector that was placed 12 cm away from the tip of the needle.Characterization of Ch-NpHydrodynamic particle size and surface chargeThe hydrodynamic size and surface charge of LMW-Ch and the electrosprayed Ch-Np were evaluated using the dynamic light scattering (DLS) method using Zetasizer Nano instrument, Nano S90 (Malvern Instruments, England).The samples were prepared by suspending 0.005 g of LMW-Ch or electrosprayed Ch-Np in 1 mL of deionized water. The samples were vortexed for 2 min to assure complete dispersion and distribution of the materials before testing. For the analysis, 1 mL of each sample was placed in a UV-transparent disposable low volume cuvette. The scattered light was collected at an angle of 90° at 25 °C. The measurements were run in triplicate.Scanning electron microscopy (SEM)A small amount of the synthesized Ch-Np powder (experimental group) and LMW-Ch (control group) was placed on a stub coated with carbon, and the samples were then coated with a thin gold film. SEM images were taken at different magnifications and at various points of the samples using a Zeiss Gemini Auriga Scanning Electron Micro analyser, equipped with a CDU-led detector at 3.00 kV with a tungsten filament to determine the morphology and particle size of the electrosprayed Ch-Np.Fourier-transform infrared spectroscopyThe structural configuration of the electrosprayed chitosan was evaluated by Fourier-transform infra-red (FTIR) spectroscopy using a Spectrum 400 FTIR/FT-NIR Spectrophotometer equipped with a universal ATR sampling accessory by PerkinElmer (PerkinElmer, Lantrisant, United Kingdom) to confirm the presence of the main functional groups of chitosan.A drop of TFA and 0.1 g of the electrosprayed chitosan, LMW-Ch were placed on the sample holder (crystal) of the PerkinElmer Spectrum 400 FTIR/FT-NIR Spectrophotometer. Each of the samples was evaluated separately by collecting the signals in 32 scans of the infrared spectra within a range of 4000–650 cm-1 in transmittance (%) at room temperature.Antimicrobial assaysThe antimicrobial activity was evaluated against planktonic cells of microorganisms related to root canal infection, namely Streptococcus mutans (ATCC 25175) (American Type Culture Collection, Manassas, VA, USA), Enterococcus faecalis (ATCC 29212), and Candida albicans (ATCC 90028). All microbial species were incubated in brain heart infusion broth (BHI) (Merck, Sigma Aldrich, Saint Louis, MO, USA) at 37 °C for 24 h. Cells from each microbial species were then suspended in phosphate buffer saline solution (PBS) (Merck, Sigma Aldrich, Saint Louis, MO, USA) and their concentrations were adjusted to 0.5 McFarland standard (Mcf) using DensiCHEK Plus (BioMérieux, Durham, NC, USA).Assessment of antimicrobial activity of LMW-ChThe first step was to determine the antimicrobial activity of LMW-Ch against planktonic cells of S. mutans, E. faecalis, and C. albicans. The commercially supplied LMW-Ch was prepared into two concentrations, namely 1% and 3%, by dispersing 10 mg and 30 mg of chitosan, respectively, in 1 mL of 3% (v/v) acetic acid26. The selection of these two concentrations was chosen as the concentration of the chitosan increased the viscosity of the hydrogel beyond 3%27, which rendered it difficult to utilize.The antimicrobial activity was evaluated using a Time-kill Test performed using the broth microdilution technique. A volume of 100 µL of each chitosan solution (1% or 3%) was dispensed in a sterile well of a 12-well cell culture plate, to which 200 µL of 0.5 (Mcf) from each tested microbial species suspension and 1700 µL of brain heart infusion broth (BHI) as a growth medium was added. The effect of acetic acid was evaluated as control by dispensing 100 µL of the acetic acid instead of the chitosan suspension. This was labelled as a negative control, while each microorganism's normal growth rate was considered a positive control group. Each group was incubated at 37 °C for 24 h. At zero minutes, 30 min, 1, 2, 4, 6, 8, and 24 h, 100 µL was removed from each group, serially diluted and plated onto freshly poured brain heart infusion agar plates. After a 24 h incubation period, the number of colony forming units (CFU) in each plate were counted using an automated colony counter (Gerber, Lyss, Switzerland). The number of colony forming units that exceeded 300 were considered as too numerous to count (TNTC) and recorded as 300 (CFU), while those < 30 were considered as too low to count (TLTC) and recorded as zero and considered as insignificant to produce illness28. The test was repeated in triplicate (3 independent experiments on separate days, each with three repeats for each organism) following the clinical and laboratory standard institute standards for dilution antimicrobial susceptibility testing29.Assessment of antibacterial activity of Ch-NpAfter establishing a baseline for the antimicrobial activity of LMW-Ch against endodontic pathogens, the second step was to evaluate and compare the antimicrobial activity of Ch-Np against planktonic cells. Furthermore, the effect of Ch-NP was evaluated against the biofilm biomass of the microbial species.The synthesized Ch-Np were allowed to completely disperse as colloids in distilled water to form a final concentration of 3% (w/v). The concentration was selected to be comparable to the maximum concentration used in LMW-Ch. The mixture was placed in a 3 mL microtube and mixed using an Eppendorf Thermomixer 5350 Mixer (Marshall Scientific, Hamburg, Germany) for 10 min at 700 rpm to allow complete dispersion.Antimicrobial activity of Ch-Np on planktonic microbial cellsThe antimicrobial activity of Ch-Np was evaluated against planktonic cells of S. mutans, E. faecalis, and C. albicans using the same method as was used to evaluate the antimicrobial activity of LMW-Ch (Time-Kill test performed by the broth micro dilution technique). The results were compared to the antimicrobial activity of LMW-Ch.Antimicrobial activity of Ch-Np on biofilm biomassThe effect of the Ch-Np against the biofilm biomass of the microbial species was evaluated using a microtiter plate biofilm assay. Biofilms of S. mutans, E. faecalis, and C. albicans were allowed to grow in sterile 96 well microtiter plates by plating 50 µL of 0.5 Mcf standard of each microbial species with 150 µL of BHI for 72 h at 37 °C. The BHI was discarded, and the biofilm was washed five times with sterile phosphate buffer saline (PBS) to remove any planktonic microbial cells and unattached microorganisms. In each of the 12 wells (n = 12), 50 µL of the prepared Ch-Np solution was added, along with 150 µL BHI, and incubated at 37 °C for 24 h. In the control group, Ch-Np was replaced with PBS. After 24 h, the BHI was removed, and each well was filled with 0.1% crystal violet to allow staining of the remaining microbial species' biofilms for 10 min. The crystal violet was removed from the biofilm by adding 30% (v/v) acetic acid in each well to solubilize the crystal violet before measuring the optical density of each sample at a wave length of 540 nm using a mic
https://www.nature.com/articles/s41598-021-04322-4
Antimicrobial and cytotoxic activity of electrosprayed chitosan nanoparticles against endodontic pathogens and Balb/c 3T3 fibroblast cells