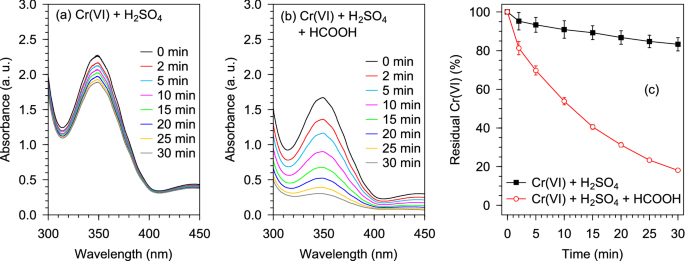
Application of polymer-coated Macadamia integrifolia nutshell biomass impregnated with palladium for chromium(VI) remediation Freely suspended and porous basket restrained granules of palladium nanoparticles supported on polymer-grafted Macadamia nutshell biomass (Pd@Polym-MNS) composite were used for the treatment chromium(VI)-containing water. In the presence of formic acid, the Pd@Polym-MNS demonstrated its activity in the adsorption-reduction-based conversion of noxious chromium(VI) to less toxic chromium(III) with a low activation energy of 13.4 kJ mol–1, ΔH0 (+ 10.8 kJ mol–1), ΔS0 (−270.0 J mol–1 K–1), and ΔG0 (+ 91.3 to + 98.0 kJ mol–1) indicated the exothermic, endergonic and non-spontaneous nature of the catalytic redox reaction. In addition to facilitating easy recovery, rinsing, and reuse, restraining the Pd@Polym-MNS in the basket reactor helped maintain the integrity of the catalysts by preventing violent collisions of suspended granules with the mixing apparatus and the walls of the reaction vessel. Whereas the pseudo-first-order rate constant was recorded as 0.157 min–1 upon initial use, values of the mean and relative standard deviation for the second, third and fourth consecutive uses were found to be 0.219 min–1 and 1.3%, respectively. According to a response surface methodological approach to batch experimentation, the initial concentration of chromium(VI) and catalyst dosage had the greatest impact on the redox reaction rate, accounting for 85.7% and 11.6% of the variability in the value of the pseudo-first-order rate constant, respectively. Mutually beneficial effects of the combinations of high formic acid and low chromium(VI) concentration, high temperature and catalyst dosage as well as high formic acid and catalyst dosage were recorded. From a health perspective, the presence of hexavalent chromium [Cr(VI)] in water is of primary concern owing to its cancer-causing effects on animal cells1. The toxicity of Cr(VI) is exacerbated by the ability of chromate ions, which structurally resemble sulfate ions, to be easily transported into cells through sulfate transporters2. Moreover, cell membranes are less permeable to trivalent chromium [Cr(III)] species3 rendering Cr(III) much less toxic than Cr(VI). Techniques based on adsorption4,5,6, solvent extraction7,8, and membrane filtration9,10 have proved to potentially provide feasible options for the removal of Cr(VI) from water. However, the main drawback associated with the removal techniques is that the pollutant often remains in its toxic hexavalent state, thereby retaining some degree of threat to the environment upon disposal of the used adsorbent, the retentate from membrane filtration, and the concentrate obtained after solvent recovery following solvent extraction. Several emerging strategies for the remediation of Cr(VI)-contaminated water, therefore, involve the transformation of Cr(VI) to Cr(III), which, for easier containment, can be precipitated as hydroxides11. Photocatalyzed Cr(VI) reduction, which allows the use of water as the reducing agent thereby eliminating the need for chemical reductants, has sparked great interest and research effort12,13,14,15. However, the costs linked to either the generation of ultraviolet light or the development of photocatalysts with high activity in the visible light (solar) region, continue to plague the use of photocatalysis in Cr(VI) remediation.The reduction of the Cr(VI) in, for example, aqueous hydrogen chromate (HCrO4–) ions by formic acid (HCOOH), which proceeds as per Eq. (1)16,17, is associated with positive electrode potentials indicating thermodynamic feasibility. However, the Cr(VI)-HCOOH redox reactions are still hampered by high activation barriers, which necessitate the use of a catalyst18. Heterogeneous catalysis in the Cr(VI)-HCOOH redox system often exploits the decarboxylation of HCOOH, which provides adsorbed hydrogen atoms (Hads) in accordance with Eq. (2) 19. The adsorbed hydrogen atoms subsequently donate their electrons to adsorbed Cr(VI) ions as exemplified by Eq. (3) 20,21. Recent reports detail the catalytic activity of materials based on different precious metals in the dehydrogenation, including gold, silver, rhodium, platinum, and palladium22,23,24,25,26. Although the activity of less costly alternatives such as nickel16,20, cobalt27, and sulfur28 has previously been reported, their use is impeded by easy leaching in the acidic HCOOH solution, resulting in poor reusability.$${{text{2HCrO}}_{4}}^{-} + text{3HCOOH+} ,{text{8H}}^{+} , rightarrow, text{2}{text{Cr}}^{3+} , + {text{3CO}}_{2}, +,{text{8H}}_{2}{text{O}} quad ,,, {text{E}}^{theta}= 1.48text{ V} ,$$$$text{HCOOH } , rightarrow, {text{CO}}_{2}, +,{text{2H}}_{text{ads}}$$$${{{text{HCrO}}_{4}}^{-}}_{text{ads}}, +,{text{3H}}_{text{ads}}, +,{text{4H}}^{+} , rightarrow, {{text{Cr}}^{3+}}_{text{ads}} , + {text{4H}}_{2}{text{O}}$$$${{text{HCrO}}_{4}}^{-}, +,{text{Fe}}^{0}, +,{text{7H}}^{+} , rightarrow, {text{Cr}}^{3+} , + text{ } {text{Fe}}^{3+}, +,{text{4H}}_{2}{text{O}}$$$${{text{HCrO}}_{4}}^{-}text{ + 3}{text{Fe}}^{2+}, +,{text{7H}}^{+} , rightarrow, {text{Cr}}^{3+} , + text{ 3} {text{Fe}}^{3+}, +,{text{4H}}_{2}{text{O}}$$$$text{HCOOH },rightarrow, {text{CO}}_{2}, +,{text{H}}_{2}$$Based on the relatively low cost of iron-based materials, several studies suggest that the use of zerovalent or divalent iron as reducing agents for the transformation of Cr(VI) to Cr(III) provides viable options for Cr(VI) remediation29,30,31,32,33,34,35,36. However, as illustrated by Eqs. (4) and (5), the resulting waste contains aqueous iron(III) species, which also precipitate alongside the Cr(III) thereby yielding large volume of sludge thus limiting the efficiency and applicability of iron-based chemical reduction techniques. Moreover, due to contamination by pure and mixed hydroxides, Fe(OH)3 and CrxFe1−x(OH)3, the precipitated chromium(III) hydroxide has lower value than purer forms formed by precipitation from wastes devoid of iron(III) species. Since the use of HCOOH as the reducing only produces carbon dioxide and water, waste solutions devoid of iron(III) species can be produced. Furthermore, excess HCOOH in the reaction mixture can be decomposed on the surface of the catalyst to liberate hydrogen gas (H2), an environmentally friendly fuel, in accordance with Eq. (6)37,38, and the decomposition of excess HCOOH lowers the acidity of the solution prior to the precipitation of chromium(III) hydroxide. Therefore, the elevated costs associated with the precious metal-based catalysts used in the Cr(VI)-HCOOH redox system can be offset by the value of the hydrogen gas and the pure chromium(III) hydroxide that can be produced via subsequent treatment with sodium hydroxide, calcium hydroxide or magnesium oxide39,40.Even though the use of nanoparticles results in enhanced catalysis, the recovery of the nanoparticles for reuse is often difficult and poses the risk of release into the environment together with the treated water. Therefore, the catalytic nanoparticles used in the HCOOH-mediated reduction of Cr(VI) have been immobilized on powders, granules, and films16,17,41,42,43,44,45,46,47. Despite several materials being used as supports for catalytic nanoparticles used in the HCOOH-mediated reduction of Cr(VI), the use of lignocellulosic material supports is scarcely reported in the literature. Following the synthesis and characterization of palladium nanoparticles dispersed on polymer-grafted Macadamia nutshell (Pd@Polym-MNS) granules in our previous work48, we have restrained the Pd@Polym-MNS granules in a porous stainless steel basket. The practical implication of restraining the catalytic Pd@Polym-MNS granules in a porous basket is that it enables easy application and recovery by immersion and retrieval, respectively, without the need for elaborate, time consuming steps in industrial wastewater treatment processes.The preparation and characterization of the composite comprising polymer-coated Macadamia integrifolia nutshell biomass with embedded Pd nanoparticles (Pd@Polym-MNS) were as described in our recent report 48. Waste Macadamia nutshells were kindly donated by the Eastern Produce Estates SA (Pty) Ltd, Tzaneen, South Africa and all experiments were performed in accordance with relevant guidelines and regulations. All reagents were sourced from Johannesburg, South Africa. Potassium dichromate (K2Cr2O7) and formic acid (HCOOH, 85 wt%) were purchased from Merck Chemical Company, and Rochelle Chemicals, respectively. Sodium sulfate (Na2SO4), sodium chloride (NaCl), and sodium nitrate (NaNO3) were obtained from Sigma-Aldrich. Sulfuric acid (H2SO4, 98% wt%) and hydrochloric acid (HCl), 32% (w/w) were purchased from Associated Chemical Enterprises. A stock solution of Cr(VI) was prepared by dissolving the K2Cr2O7 in deionized water obtained from a Milli-Q water system (Milli-Q Direct, with 18.2 MΩ cm resistivity, Merck Millipore Corporation, USA).Cr(VI) conversion experimentsThe experiments to assess the conversion of Cr(VI) by reaction with HCOOH were carried out in three batches. In the first batch of experiments, mixed Cr(VI)-HCOOH solutions were prepared by diluting mixtures of the Cr(VI) stock solution, H2SO4 and HCOOH with deionized water. Thereafter, the solution was incubated at a specific temperature, the Pd@Polym-MNS was added, and the suspension was magnetically stirred throughout the experiment. A porous basket reactor comprising a 40 mesh stainless steel cylindrical basket into which Pd@Polym-MNS was weighed before covering with a shafted lid was used in the second and third experimental batches. In the second batch of Cr(VI) conversion experiments, the Pd@Polym-MNS-loaded basket was immersed in an incubated mixed Cr(VI)-HCOOH solution prepared by the dilution of mixtures of the Cr(VI) stock solution, HCOOH, H2SO4, NaCl, and NaNO3 with tap water. The basket was either kept stationary as the solution was stirred using an overhead paddle or the solution was stirred by rotation of the basket reactor on a dissolution testing apparatus (Vision G2 Classic 6, Teledyne Hanson Research, Chatsworth, CA, USA). In order to facilitate the analysis of the combined influence of initial Cr(VI) concentration (X1), initial HCOOH concentration (X2), Pd@Polym-MNS dose (X3), and temperature (X4) on the rate of Cr(VI) conversion, the third batch of Cr(VI) conversion experiments was conducted as per a 24 factorial central composite experimental design with the coded factor levels and values of the input or independent variables presented in Table 1.Table 1 Input variable codes, levels, and values of the central composite design-based experiment.Using the rate constant of the model providing the best fit to the Cr(VI) conversion kinetics as the response or dependent variable (Y), the relationship between the response and input variables was modeled using a quadratic equation expressed as:$${text{Y}}=beta_{0}, +,beta_{1}{{text{X}}}_{1}, +,beta_{2}{{text{X}}}_{2}, +,beta_{3}{{text{X}}}_{3}, +,beta_{4}{{text{X}}}_{4}, +,beta_{12}{{text{X}}}_{1}{{text{X}}}_{2}, +,beta_{13}{{text{X}}}_{1}{{text{X}}}_{3}, +,beta_{14}{{text{X}}}_{1}{{text{X}}}_{4}, +,beta_{23}{{text{X}}}_{2}{{text{X}}}_{3}, +,beta_{24}{{text{X}}}_{2}{{text{X}}}_{4}, +,beta_{34}{{text{X}}}_{3}{{text{X}}}_{4}, +,beta_{11}{{text{X}}_{1}}^{2}, +,beta_{22}{{text{X}}_{2}}^{2}, +,beta_{33}{{text{X}}_{3}}^{2}, +,beta_{44}{{text{X}}_{4}}^{2} ,$$where Y represents the predicted response; β0 the offset term; β1, β2, β3, and β4 the linear coefficients; β12, β13, β14, β23, β24 and β34 the interaction coefficients; and β11, β22, β33, and β44 the quadratic coefficients. X1, X2, X3, and X4 represent the input variables as defined in Table 149. Using Statistica software (Version 10.0, StatSoft, Inc., Tulusa, OK, USA), Eq. (7) was fitted to the experimental data, and surface plots were created to evaluate the combined effects of the input variables.At specific times during the course of each Cr(VI) conversion experiment, a 3.5 mL sample of the working solution was transferred into an optical quartz cell with a 1 cm path length and its ultraviolet–visible (UV–Vis) spectral absorption was recorded on a UV–Vis spectrophotometer (Cary 60, Agilent Technologies, Santa Clara, CA, USA) over a wavelength range of 300 nm to 500 nm at a scanning rate of 1200 nm min–1 with increments of 2 nm and a step time of 0.10 s. Immediately afterward (approximately 2 min after sampling), the sample was returned to the reaction mixture. The evolution of Cr(VI) concentration was monitored by measurement of the absorbance of the peaks at the characteristic wavelengths between 350 and 375 nm50. In accordance with the Beer-Lambert Law, which states that spectral absorbance is directly proportional to concentration, the absorbance of the Cr(VI) solutions at the beginning of each Cr(VI) conversion experiment and at any time t thereafter, denoted A0 and At (arbitrary units), respectively, was related to the Cr(VI) concentration at the beginning of each experiment and at any time t during the experiment, denoted where [Cr(VI)]0 and [Cr(VI)]t (mmol L–1), respectively, in accordance with Eq. (8). The fraction of Cr(VI) that remained in the solution any time during the experiment, designated Residual Cr(VI) (%), was calculated using Eq. (9).$$frac{{text{A}}_{text{t}} , }{{text{A}}_{0}} = frac{{text{[Cr(VI)]}}_{text{t}}
https://www.nature.com/articles/s41598-021-03473-8
Application of polymer-coated Macadamia integrifolia nutshell biomass impregnated with palladium for chromium(VI) remediation