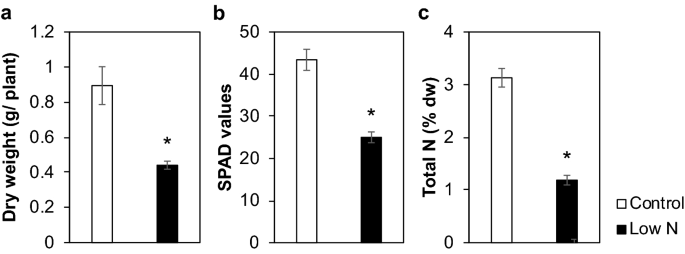
Nitrogen deficiency results in changes to cell wall composition of sorghum seedlings Sorghum [Sorghum bicolor (L.) Moench] has been gaining attention as a feedstock for biomass energy production. While it is obvious that nitrogen (N) supply significantly affects sorghum growth and biomass accumulation, our knowledge is still limited regarding the effect of N on the biomass quality of sorghum, such as the contents and structures of lignin and other cell wall components. Therefore, in this study, we investigated the effects of N supply on the structure and composition of sorghum cell walls. The cell walls of hydroponically cultured sorghum seedlings grown under sufficient or deficient N conditions were analyzed using chemical, two-dimensional nuclear magnetic resonance, gene expression, and immunohistochemical methods. We found that the level of N supply considerably affected the cell wall structure and composition of sorghum seedlings. Limitation of N led to a decrease in the syringyl/guaiacyl lignin unit ratio and an increase in the amount and alteration of tissue distribution of several hemicelluloses, including mixed linkage (1 → 3), (1 → 4)-β-d-glucan, and arabinoxylan. At least some of these cell wall alterations could be associated with changes in gene expression. Nitrogen status is thus one of the factors affecting the cell wall properties of sorghum seedlings. A transition from fossil fuels to renewable energy has been considered the key to attaining energy security and environmental sustainability. As of 2018, renewable energy accounted for approximately 14% of the global primary energy supply1, and this share is expected to grow further2. Bioenergy is a sector of renewable energy that includes municipal waste, industrial waste, solid biofuels, biogases, and liquid biofuels, and it accounts for approximately 67% of the current renewable energy mix1. Hence, increased production of bioenergy feedstocks would be significant to facilitate the shift to renewable energy, in turn, stimulating the demand for biomass as the feedstock for bioenergy.Sorghum [Sorghum bicolor (L.) Moench] is a multi-purpose crop that delivers food, fodder, and fibers3,4,5. Recently, it has received increasing attention as a raw material for bioenergy6,7,8,9,10,11,12,13. The strategy for converting sorghum into energy depends on the type of sorghum7. Grain sorghum produces starchy grains that can be a cost-effective source of starch used in ethanol production12. Sweet sorghum accumulates sugar in its stalk, which can also be utilized for fermentation. In addition, biomass sorghum can produce more than 42 t ha−1 biomass under favorable conditions11, which can be utilized for solid biofuel applications such as biopellets or biochar7,8,9,10. Another advantage of sorghum is its adaptability to adverse conditions, such as drought or salinity14. This characteristic makes it possible to grow sorghum on land that is not suitable for the cultivation of other crops such as rice or maize, as a way to produce biomass feedstock without encroaching on existing arable lands used for food production.One constraint for crop production in marginal lands is low soil fertility. Among the plant nutrients taken up from the soil, nitrogen (N) is required in the greatest quantities and hence often becomes a growth-limiting factor. The impact of N supply on sorghum biomass accumulation has been well documented15,16,17,18,19. However, information on the effect of N deficiency on the quality of sorghum biomass is limited. The quality of biomass as a feedstock for energy production depends on its composition. Grass cell walls are mainly composed of cellulose, hemicelluloses, and lignin. Cellulose, the crystalline aggregate of (1 → 4)-β-d-glucans, is the most abundant component of grass cell walls. Hemicelluloses are associated with cellulose as crosslinks to form the cellulose-hemicellulose network as the framework of the cell walls. Major hemicelluloses in grasses include non-crystalline (1 → 4)-β-d-glucans, mixed linkage (1 → 3), (1 → 4)-β-d-glucan (MLG), arabinoxylan, glucuronoxylan, and xyloglucan20. Lignin is a phenylpropanoid polymer that fortifies cell walls by filling up the spaces between the polysaccharides21,22,23 and is biosynthesized via oxidative coupling of p-hydroxycinnamyl alcohols (monolignols) and related compounds formed in the cinnamate/monolignol pathway24,25. Lignin is composed of three major units: syringyl (S), guaiacyl (G), and p-hydroxyphenyl (H), which are formed by the polymerization of sinapyl, coniferyl, and p-coumaryl alcohols, respectively24,25. Grass lignin, especially in S units, is often acylated with p-coumarate21,26,27. Moreover, a flavone, tricin, has been found to serve as a lignin monomer in grasses26,28,29,30. Ferulate is also abundant in grass cell walls and is thought to serve as a crosslink among hemicelluloses and between lignin and hemicelluloses31.In general, lignin content is positively correlated with the heating value of lignocellulose32; therefore increased lignin content can be an advantage when the cell wall, or bagasse, is considered as a source of solid biofuels 13,23,30,33. On the other hand, higher lignin content can lead to higher cell wall recalcitrance against enzymatic digestion21. Hence, it is important to understand the effects of N nutrition on lignin and other cell wall components in biomass crops. There have been several reports on the impact of N supply on cell wall composition in grasses. For example, lignin content was decreased by N fertilizer application in rice (Oryza sativa)34, maize (Zea mays)35, and brachypodium (Brachypodium distachyon)36. By contrast, N fertilizers increased the lignin content in cell walls of giant miscanthus (Miscanthus × giganteus)37. These findings suggest that the effect of N-supply on cell wall components differs among plant species. In sorghum, a recent report38 has shown that application of N increased the lignin content in sweet sorghum cultivated in a semi-arid environment. However, it remains to be investigated precisely how each cell wall component can be affected by N supply, and by what mechanism the change is induced in sorghum. Therefore, in this study, we investigated N deficiency-induced changes in cell walls of sorghum seedlings, with the aim of determining early effects of different N status on sorghum cell wall structure. The study was conducted using a hydroponic culture system to minimize the intervention by other factors, and the properties of cell walls from N-deficient plants and those from plants receiving sufficient amounts of N were compared through chemical, two-dimensional (2D) heteronuclear single quantum coherence (HSQC) nuclear magnetic resonance (NMR), gene expression, and immunohistochemical analyses.Sorghum seedlings were grown hydroponically using a standard culture medium and a medium containing a low level of N (1/10th), as control and low-N treatments, respectively. The low-N treatment reduced the dry weight of seedlings by 51% (Fig. 1a), with decreased chlorophyll and N contents (Fig. 1b,c). The impact of low-N treatment on cell wall properties was then examined by chemical analyses. Thioglycolic acid lignin analysis suggested that N supply did not significantly affect lignin content (Table 1). However, the lignin aromatic composition was affected by N supply, as there was a 27% decrease in the thioacidolysis-derived syringyl/guaiacyl-type monomer ratio (S/G unit ratio) in low-N plants, with a 48% increase in the G-type monomer composition, as compared to the control plants (Table 1). The contents of cell wall-bound hydroxycinnamates, including ferulic acid (FA) and p-coumaric acid (pCA), were also analyzed. The content of cell wall-bound pCA, which is mainly attached to lignin39,40, did not differ between control and low-N plants. On the other hand, the content of FA, which is mainly attached to arabinoxylan 27,40,41,42, was increased by 56% under low-N conditions (Table 1).Figure 1Effect of low-N treatment on hydroponically-grown sorghum seedlings growth. (a) Dry weight, (b) soil plant analysis development (SPAD) values, and (c) N content of hydroponically-grown sorghum seedlings cultivated under control or low-N conditions at 3 weeks after treatment. Values are means ± SD (n = 3). Asterisks indicate significant differences between control and low-N plants. (Student’s t test, p < 0.05).Table 1 Cell wall chemical analysis of hydroponically-grown sorghum seedlings cultivated under control and low-N conditions at 3 weeks after treatment. Asterisks indicate significant differences between low-N and control plants (Student's t test, p < 0.05). CWR: cell wall residue, H: p-hydroxyphenyl, G: guaiacyl, S: syringyl, pCA: p-coumarate, and FA: ferulate, TFA: trifluoroacetic acid. Values are means ± SD (n = 3).Regarding the polysaccharide fraction, the content of crystalline cellulose was higher in low-N plants, but the difference was not statistically significant (Table 1). Analysis of the glycosyl residue composition of the trifluoroacetic acid (TFA)-soluble, non-crystalline polysaccharide fraction indicated that the low-N plants contained more arabinosyl, xylosyl, galactosyl, and glucosyl residues than the control plants (Table 1). The glucosyl residue content increased more than twofold upon low-N treatment. We also estimated changes in pectins, another class of cell wall polysaccharides, by quantifying uronic acids, and calcium (Ca) possibly bound to pectin. The uronic acid and Ca contents were decreased by 18% and 45% in low-N plants, respectively (Table 1).Methylation analysis revealed the presence of at least 16 glycosyl residues with different linkages in the cell walls of sorghum seedlings (Table 2). Of the sugar residues listed in Table 1, mannose, which was probably derived from mannans, remained unaffected by the low-N treatment. Therefore, we estimated the amount of each detected residue relative to 4-linked mannosyl. The glycosyl residues that were increased by low-N treatment included 3- or 4-linked glucosyl and 4- or 3,4-linked xylosyl residues (Table 2). In grass cell walls, the 3-linked glucosyl residue is found in callose [(1 → 3)-β-d-glucan] and MLG, whereas the 4-linked glucosyl residue in non-crystalline (1 → 4)-β-d-glucan and MLG. The 4- and 3,4-linked xylosyl residues are found in xylans with or without substitution, such as xylan, arabinoxylan, or glucuronoxylan. The observed increase of these residues suggests that the cell walls of low-N plants contained more hemicelluloses and/or callose. Galactosyl residues were found to be increased in the composition analysis (Table 1) but not in the methylation analysis (Table 2) of the glycosyl residue. The reason for this apparent discrepancy is unclear at present.Table 2 Glycosyl linkage composition of the cell walls of hydroponically-grown sorghum seedlings cultivated under control and low-N conditions at 3 weeks after treatment. Relative abundance of the residues was calculated as molar ratio relative to 4-linked mannosyl residue. The averages of duplicate (control) or triplicate (low-N) determinations are shown. Values are the average of two (control) or three (low N) replicate samples.Nuclear magnetic resonance analysisTo further investigate the low N-induced alterations of the sorghum cell wall structure, we performed 2D HSQC NMR analysis on the cell wall samples from the sorghum seedlings using the NMR approach43,44. The aromatic sub-regions of the obtained HSQC NMR spectra displayed well-resolved contour signals from the lignin aromatic units such as S (S), G (G), and tricin (T) units, along with the signals from the hydroxycinnamate FA (F) and pCA (P) units (Fig. 2a). In addition, the sugar anomeric sub-regions of the spectra displayed contour signals from cell wall polysaccharide components, including glucan (Gl), non-acetylated (Xy), and acetylated (Xyʹ and Xyʹʹ) xylan, arabinan (Ar), galactan (Ga), and glucuronan (GU) units (Fig. 2b). To estimate the structural differences between the control and low-N sorghum cell walls, these contour signals were integrated and normalized based on the sum of the S and G lignin aromatic signals (S + G) (Fig. 2c,d).Figure 2Two-dimensional short-range 1H-13C correlation nuclear magnetic resonance (2D HSQC NMR) spectra of the cell walls of hydroponically-grown sorghum seedlings cultivated under control and low-N conditions at 3 weeks after treatment. The NMR spectra were acquired with composite samples prepared from three replicates. (a) Aromatic sub-regions showing signals from major lignin hydroxycinnamate aromatic units. Contours are color-coded to match the displayed structures. Boxes labeled × 2 means regions with scale vertically enlarged twofold. (b) Anomeric sub-regions showing signals from major cell wall polysaccharide units. Py, pyridine (solvent). Phe and Tyr, phenylalanine and tyrosine residues in residual proteins45. (c) Normalized intensity of major lignin, hydroxycinnamate and polysaccharide signals expressed on a S + G = 1 basis. Data labeled × 1/10 indicate that the reported values are divided by a factor 10 for visualization purposes. (d) S/G signal ratio.The S (S) and G (G) lignin signals were relatively decreased and increased, respectively, in the HSQC spectrum of the low-N sorghum cell walls compared to those in the HSQC spectra of the control cell walls (Fig. 2c). Consequently, the S/G signal ratio was notably reduced in the low-N cell wall spectrum (0.13) compared to that in the control cell wall spectrum (0.26) (Fig. 2d). This result was in accordance with the significant reduction in the S/G monomer ratio, as determined by thioacidolysis (Table 1). In addition, the FA (F) signals were notably increased in the low-N cell wall spectrum, corroborating the chemical analysis data th
https://www.nature.com/articles/s41598-021-02570-y
Nitrogen deficiency results in changes to cell wall composition of sorghum seedlings