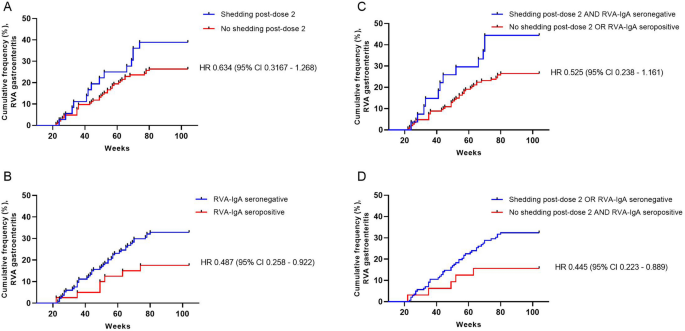
As assessed among young children in Dhaka, Bangladesh, lack of RVA shedding following the second dose of Rotarix appeared to be associated with protection from subsequent RVA diarrhea through 2 years of life, although outcomes of some analyses did not reach strict statistical significance. To our knowledge, these are the first data that evaluate vaccine shedding in the context of a CHIM coupled with RVA diarrhea incidence and not simply as a measure of potential vaccine take. Immunity from RVA gastroenteritis is unlikely to require sterilizing immunity, meaning that protection from diarrhea may occur despite asymptomatic or mild infection16,17,18. However, our results suggest that protection from asymptomatic infection by vaccine-strain virus in a human challenge model may yet be an informative surrogate for clinical protection from RVA gastroenteritis. While the directions of the effects observed in our dataset were clear, the precision of our estimates was low due to limitations in sample size, as evidenced by our large confidence intervals, which in some analyses included 1 (indicating failure to meet strict statistical significance), and by the timing of sample collection. Therefore, these data require further confirmation in larger prospective studies. Nevertheless, the results suggest that CHIMs utilizing approved oral rotavirus vaccines warrant further development, particularly in contexts where the standard immunogenicity measure RVA-IgA may not be an appropriate outcome, such as with next-generation, non-replicating vaccines19.
Lack of association between post-dose 2 shedding and plasma RVA-IgA seropositivity suggests that lack of shedding may detect mucosal immunity that may not be always be reflected in plasma/serum antibody measurement. Nevertheless, inhibition of fecal shedding in our model did not appear to offer any appreciable advantage over RVA-IgA, which is currently the best available CoP for RVA, despite its limitations3,20. However, the RVA-IgA assay predominantly measures antibodies against the RVA middle capsid structural protein VP6, which is the immunodominant antigen in the humoral response to RVA. VP6 is available for recognition only after successful infection of host intestinal cells leads to uncoating of the outer capsid layer to reveal VP6 and initiate viral replication21. Therefore, alternate assays are likely needed to adequately assess immune responses for next-generation, non-replicating vaccines that use antigens other than RVA VP619. Rotarix shedding has been used in phase 1 and 2 trials of a P2-VP8* parenteral vaccine candidate to evaluate for induction of mucosal immunity22,23. In a phase 1/2 safety and immunogenicity trial of the trivalent P2-VP8* formulation, infants who received the highest dose of 90 ug had significantly reduced rates of PCR-confirmed Rotarix shedding following the first Rotarix dose compared to infants who received placebo22. A phase 3 efficacy trial of this vaccine is currently in progress (Clinicaltrials.gov NCT04010448), but no data currently exist regarding vaccine shedding and vaccine efficacy for parenteral vaccines.
Our results likely underestimated the potential impact of inhibition of vaccine shedding, since we were only able to assess vaccine shedding following the second dose of the vaccine, and not at a subsequent time point after completion of the full vaccine series. Based on RVA-IgA immunogenicity data, early studies from Latin America and Finland suggest that anywhere from 8 to 23% of vaccinated infants did not respond to the first dose of vaccine but responded to the second (our calculation)12,13. Thus, in this study a subset of children who did not respond to the first dose of Rotarix may have responded to the second dose and shed vaccine. If so, they may have acquired immunity to future infection due to the second dose of vaccine and may have inhibited RVA shedding if challenged with an additional dose at a later time point, meaning such children would have been misclassified here, even if the total number likely comprised a relatively small proportion of participants. Another factor that may have led to underestimate of immune response is measurement of antibody response 1 week after the second dose, which would have reflected first-dose response, but may have been too early to capture second-dose immune responses, as explained in further detail elsewhere20.
It is also possible that inhibition of post-dose 2 shedding was due to factors other than a response to the first vaccine dose. For example, high levels of maternally derived, RVA-specific serum IgG antibodies are associated with decreased oral rotavirus vaccine immunogenicity20,24,25. Infants who inhibited vaccine shedding due to high maternal antibodies may have become susceptible to RVA infection later in life as these antibodies waned in the absence of an adequate vaccine response to replace them. Unfortunately, only a subset of infants in our parent study had pre-vaccination RVA-specific IgG (RVA-IgG) antibodies measured, such that only 59 infants had both post-dose 2 shedding and pre-vaccination RVA-IgG data available for analysis; this small sample size precluded meaningful analysis.
We previously attempted to consider dose 1 response by evaluation of post-dose 1 shedding, and reported that post-dose 1 shedding as a marker for successful vaccine take or post-dose 1 shedding followed by lack of shedding post-dose 2 was not associated with subsequent year 1 RVA diarrhea19,26. However, among infants with post-dose 1 shedding specimens available for analysis (N = 176), 73% had specimens collected within 2 days following vaccination, meaning many detections at this time point could reflect passage of vaccine inoculum and not shedding indicative of viral replication, confounding interpretation of results relying on post-dose 1 shedding as a marker for successful vaccine take. Similarly, the possibility that detection of shedding post-dose 2 in some children could merely indicate passage of vaccine inoculum, rather than vaccine replication, cannot be completely ruled out.
In summary, infants in Dhaka, Bangladesh who inhibited fecal shedding of Rotarix, a live-attenuated, oral vaccine, following the second vaccine dose demonstrated a clear trend towards reduced risk for subsequent RVA diarrhea through 2 years of life. Based on these data, further development of novel CHIMS using approved oral rotavirus vaccines as challenge agents to explore immune COPs for RVA appears warranted, particularly for the evaluation of next-generation, non-replicating rotavirus vaccines.
http://feeds.nature.com/~r/srep/rss/current/~3/H-M435JNFjA/s41598-021-01288-1